Fig. 6 |. Ubiquitin and ubiquitin-like modifier ligase-mediated isopeptide bond formation and its applications.
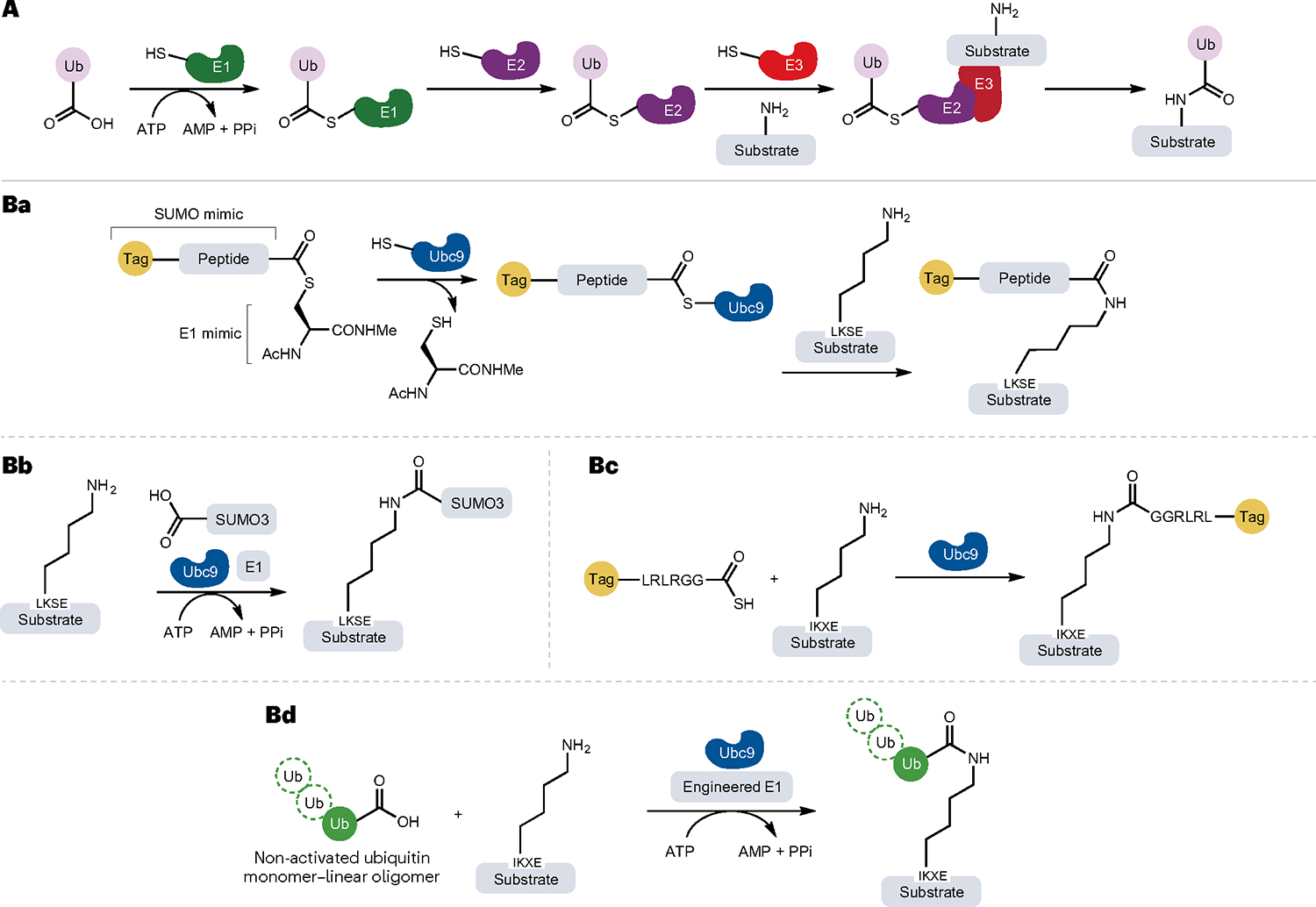
A, General mechanism of protein ubiquitylation (isopeptide bond formation) catalysed by E1, E2 and E3 enzymes. Ba,Bc, Site-specific modification and ubiquitylation of target proteins containing synthetic or genetically encoded tags by the LACE platform (lysine acylation using conjugating enzymes). A minimal genetically encoded tag (LKSE or IKXE) in the substrate proteins or peptides acts as acyl acceptor, whereas a peptide sequence (LRLRGG) mimicking the C terminus of ubiquitin (Ub) functions as an acyl donor when functionalized as a thioester. Thus, the thioester acyl donor mimics the structure of the small Ub-like modifier (SUMO)–E1 complex. Using thioesters and Ub-conjugating enzyme E2 (Ubc9), proteins can be modified with chemical probes and even small proteins. Bb, Non-activated SUMO3 can be site specifically installed at the LACE tag by the combined action of Ubc9 and an E1 enzyme in the presence of the cofactor ATP, which is hydrolysed to AMP and two molecules of phosphate (PPi). Bd, The development of a chimeric E1 enzyme enables conjugation of non-activated Ub. Ac, acetyl group; Me, methyl group.
