Fig. 8 |. Split intein-based strategies for generating semisynthetic proteins.
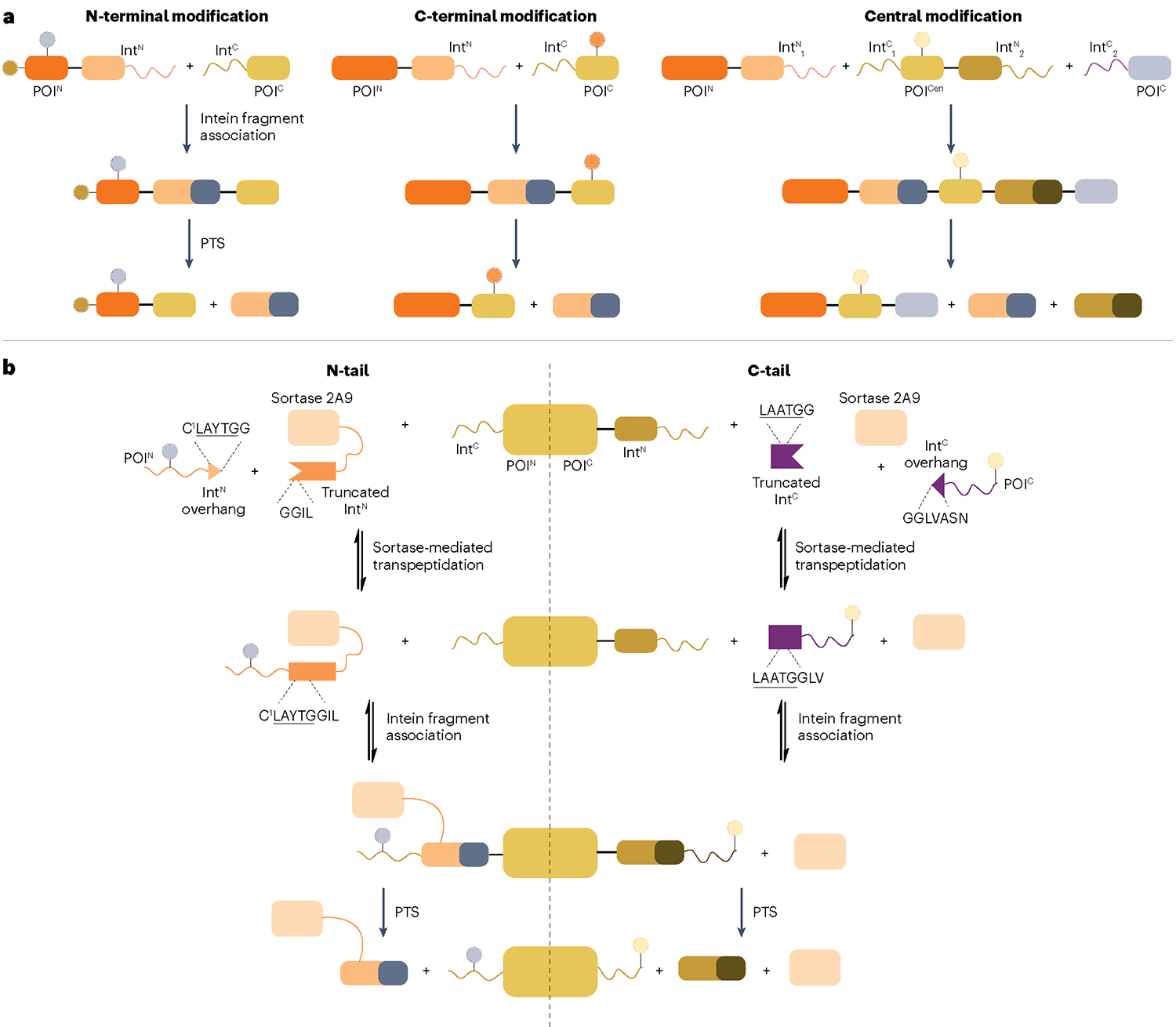
a, Proteins can be site-specifically modified at their termini by splitting them into two separate pieces, each fused to a split-intein fragment. For N-terminal modification, a short, synthetic N-terminal segment of the protein of interest (POI) (POIN) is fused to the N-terminal split intein fragment (IntN), whereas the recombinantly expressed C-terminal POI fragment (POIC) is fused to the C-terminal split-intein fragment (IntC). Upon association and protein trans-splicing (PTS), an N-terminally modified POI is produced. Similarly, C-terminal modification is performed by making the IntC–POIC complex synthetically. To modify a central segment of a POI (POICen), orthogonal intein pairs are used in a tandem protein splicing scheme in which the POICen is synthetic and fused to intein fragments at both termini. IntN1 and IntC1 as well as IntN2 and IntC2 represent two pairs of orthogonal split inteins. b, Transpeptidase-assisted intein ligation (TAIL) enables N-terminal (N-tail) and C-terminal (C-tail) modification of proteins by combining sortase- and intein-mediated protein ligation. Split inteins are further split into small overhangs (IntN overhang and IntC overhang) containing sortase recognition sequences LAYTG or LAATG and truncated, inactive inteins. The reversible, sortase-mediated transpeptidation step generates active split inteins carrying synthetic cargo, which associate with their split-intein partner for protein semisynthesis through irreversible PTS. Note that sortase and the truncated IntN are fused in N-tail to increase the reaction rate and thereby suppress premature cleavage of IntC–POIN complex.
