Figure 6. Model for the regulated activation of MurG by SpoVK.
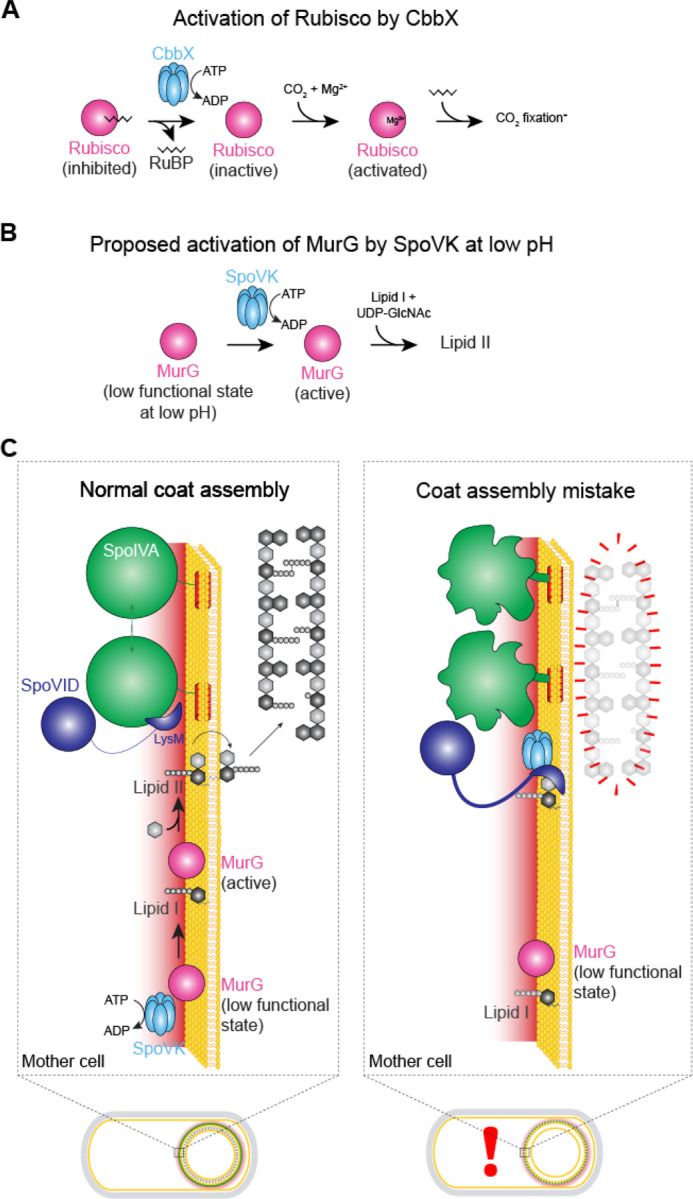
(A) Activation of Rubisco, which catalyzes fixation of CO2 in photosynthesis, by AAA+ chaperone CbbX. Rubisco (pink) forms an inactive complex with its substrate ribulose 1,5-bisphosphate (RuBP, black), which can be reactivated by CbbX (blue). Rubisco is subsequently activated by reacting with CO2 and Mg2+ (adapted from Mueller-Cajar et al. (1)). (B) Proposed activation of MurG by AAA+ chaperone SpoVK. MurG exists in a low functional state at the forespore surface due to the low pH in that nanoenvironment. SpoVK, which localizes to the forespore surface, helps fold MurG properly so that it may catalyze the conversion of lipid I to lipid II. (C) Depicted are a sporulating cell of B. subtilis that is wild type (left) or a mutant (right) that mis-assembles the spore coat. Expansions of the developing spore envelope (cortex, outer forespore membrane, and coat basement layer) are depicted above each cell. Low pH nanoenvironment is depicted as a red gradient. When SpoIVA (green) polymerizes properly, the LysM domain of SpoVID (purple) is occluded, permitting 1) lipid II to flip to the intermembrane space to incorporate into the assembling cortex and 2) SpoVK (blue) to activate MurG, thereby ensuring a steady flux of lipid II. When SpoIVA mis-assembles (right), the LysM domain of SpoVID is liberated, resulting in 1) sequestration of lipid II and 2) inhibition of SpoVK, resulting in reduced buildup of lipid II in the mother cell cytosol, due to the low functional state of MurG in the forespore surface nanoenvironment.
