FIGURE 4.
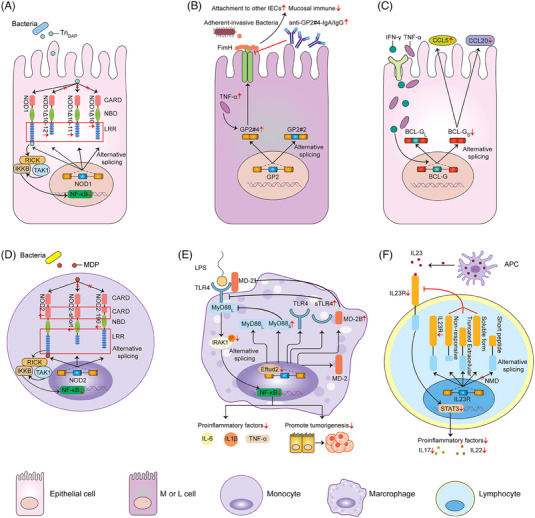
AS and innate immune. (A) In IBD, only other three splicing variants but not the full‐length of NOD1 are up‐regulated by inflammatory stimuli, thus blocking the NF‐κB pathway upon stimulation and inactivating the host responses to bacterial infection. (B) GP2‐splicing variant 4 (GP2#4) rather than variant 2 induced by TNF‐α performs as a specific receptor for FimH‐positive bacteria to induce translocation of AIEC to the below Peyer's patches and activate protective immune responses. On the contrary, serum GP2 autoantibodies inhibit the FimH binding to GP2#4, thereby leading to a corresponding reinforced attachment of flagellated bacteria to other intestinal epithelium, impairing mucosal immune and exacerbating intestinal inflammation. (C) In healthy gut tissue, both human BCL‐G splice variants (BCL‐GL and BCL‐GS) are over‐expressed in IECs. In IBD patients, increased tissue expressions of Th1 cytokines (IFN‐γ and TNF‐α) can strongly suppress the BCL‐GS expression, which differentially regulates the inflammatory chemokines (CCL5 and CCL20). (D) In the PBMCs of IBD patients, both the full‐length and alternatively spliced variants (NOD2‐short and NOD2‐190) are synchronously down‐regulated, but only the former is active and responsive to the NF‐κB. (E) Eftud2 deletion in macrophage deregulates the mRNA splicing of MyD88/TLR4/MD‐2. Alternatively spliced forms of MyD88s, MD‐2B and sTLR4 may contribute to the inhibition of the TLR4 signalling, NF‐κB activation as well as the release of some pro‐inflammatory mediators. (F) The changes of different splice variants of IL23R mediate the STAT3 pathway via the ligand‐binding interaction.
