FIGURE 5.
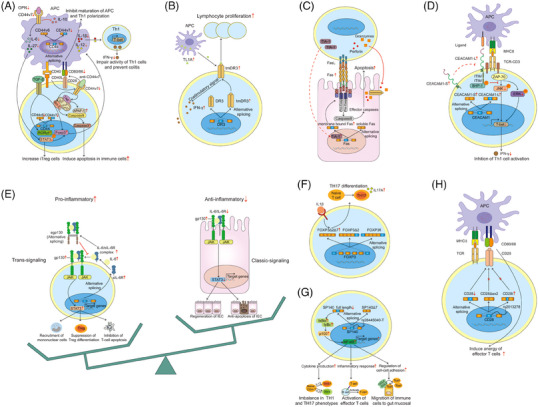
AS and adaptive immune. (A) In intestinal macrophage and T cells, blockade of CD44v6 and CD44v7 induces apoptosis in immune cells and prevent the chronic inflammation by distinct pathways. (B) Predominant expression of the tmDR3 in preference to the soluble form on lymphocytes can trigger the costimulatory signals, amplify the IFN‐γ secretion and connect to the pathogenesis of Th1‐associated inflammation. (C) In CTLs, TIA‐1 acts as an AS regulator, and generates a membrane‐bound form of FAS receptor, which can lead to more cryptal apoptosis and abscesses. (D) In mouse intestinal T cells, owing to the two phosphorylated ITIMs, up‐regulated CEACAM1‐L and CEACAM1‐S after ligand interaction mediate the TCR–CD3 pathway, and result in the inhibition of Th1 differentiation and secretion. (E) Classic IL‐6 activation via IL‐6Rs seems to play a protective role, while sIL‐6R‐mediated cell signal (IL‐6 trans‐signalling) exerts pro‐inflammatory effects. (F) IL‐1β promotes abnormal patterns of FOXP3 splicing with an up‐regulated proportion of FOXP3Δ2Δ7, which can favour the differentiation of naïve T cells into Th17 cells, and contribute to IL‐17 production and disease severity. (G) SP140Δ7 altered by rs28445040‐T inhibits the NF‐κB activity in B cells, and is involved in regulation of cytokine production, inflammatory response and cell‐cell adhesion. (H) Both CD28i and CD28Δex2 associated with ligands are confirmed as loss‐of‐function splicing isoform products that can reduce disease risk by inducing anergy of effector T cells.
