Abstract
Background
In this study, we aimed to investigate the performance of GALAD, GALAD-C, and GAAP models in Chinese population in comparison to our newly build statistical model.
Methods
In this study, we built the AALP model based on age, α-fetoprotein (AFP), AFP-L3, and prothrombin induced by vitamin K absence-II (PIVKA II) to differentiate between patients with HCC and patients with CLD. We then compared the serum levels of AFP-L3 and PIVKA II in patients with HCC who were defined as remission or progression and showed the prognostic value of combined biomarkers.
Results
The AUC value of the AALP model for HCC detection was 0.939 and AALP model exhibited a sensitivity of 81 % and a high specificity of 95 %. AALP model also exhibited good performance in the subgroups of patients with CLD. Furthermore, we demonstrated the consistency between imaging results and serum levels of AFP-L3 and PIVKA II.
Conclusions
The AALP model achieved a good diagnostic performance and a high sensitivity for predicting HCC patients. Our research also showed that AFP-L3 and PIVKA II are complementary to each other but irreplaceable in the clinical detection and monitoring of HCC.
Keywords: Hepatocellular carcinoma, Alpha-fetoprotein, Diagnosis, Prothrombin induced by vitamin K absence-II
1. Introduction
Primary liver cancer is the fifth most common malignant tumor and the second leading cause of mortality in China [[1], [2], [3]]. Primary liver cancer includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular–cholangiocarcinoma. HCC accounts for 75–85 % of all liver cancer cases and ICC accounts for 10%–20 % [4]. The number of HCC cases and mortality will increase over the next 20 years [5]. Most patients with hepatocellular carcinoma (HCC) are diagnosed at an advanced disease stage and the 5-year survival rate is only 12 % [6]. It would be beneficial to identify effective molecular diagnostic markers that will allow the early detection of cancers and improve long-term survival and patient quality of life [7].
AFP is the most utilized surveillance biomarker for screening HCC. However, the sensitivity and specificity values of AFP for HCC are only 41%–65 % and 76%–94 %, respectively [8]. AFP levels are not significantly increased in nearly 40 % of patients with HCC, whereas AFP levels are increased in patients with CLD [9,10]. Owing to the limited performance of AFP for the diagnosis of HCC in patients with CLD, AFP, and AFP-L3 combined with prothrombin induced by vitamin K absence-II (PIVKA II) were suggested to increase their diagnostic value for differentiating between patients with HCC patients and patients with CLD [11].
AFP-L3 is a glycol-forms of AFP. The other two glycol-forms are AFP-L1 and AFP-L2 [12]. AFP-L3 is more specific for HCC, whereas AFP-L1 is correlated with hepatic inflammation and AFP-L2 is correlated with pregnancy [13]. AFP-L3 is calculated as the fraction of AFP-L3 to total AFP and is usually used as an HCC diagnostic biomarker [14]. Protein induced by the absence of vitamin K or antagonist-II (PIVKA II), also known as des-gamma-carboxy prothrombin (DCP), is undetectable in healthy patients and is used as a biomarker for diagnosing HCC [15]. PIVKA II is recommended as a surveillance biomarker by the Europe Association for the Study of Liver and the American Association for the Study of liver [16]. However, previous research has shown that PIVKA-II combined with AFP increased diagnostic accuracy in HCC patients with HCV [17]. To clarify whether combined three biomarker could increase diagnostic accuracy in HCC patients, we investigated the association of combined biomarkers with diagnostic accuracy. To go a step further, we continued to consider increasing diagnostic accuracy by designing diagnostic models based on Chinese patients. Johnson et al. developed the GALAD model which is based on gender, age, and three biomarkers AFP, AFP-L3, and PIVKA II. The performance of the GALAD model has been validated in Germany and Japan but has not been evaluated in the Chinese population. Liu et al. developed GALAD-C and GAAP models based on Chinese patients. They showed that GALAD-C is superior to GALAD and GAAP models in Chinese patients. However, the effectiveness of this scoring model should be further verified in different clinical cohorts. We aimed to construct a new model based on the Chinese population. We aimed to evaluate the prognostic value of AFP, AFP-L3, and PIVKA II for the diagnosis of HCC and compare the performance of GALAD, GALAD-C, and GAAP models with our new model. In addition, we aimed to compare the consistency between serum levels of AFP-L3 and PIVKA II and imaging results included magnetic resonance imaging (MRI), computed tomography (CT), and angiography.
2. Materials and methods
2.1. Study design and sample collections
Between 2018 and 2022, we enrolled 1241 patients from the Liaocheng People's Hospital. The patients were primarily diagnosed with HCC or had chronic disease (CLD), cirrhosis, or chronic hepatitis. There were a total of 395 patients with HCC and 846 with CLD. The CLD patients were assigned to 3 subgroups in accordance with their clinical diagnosis—hepatitis carrier (HC) group, chronic hepatitis B (CHB) group, and liver cirrhosis (LC) group. The HC, CHB, and LC groups included 217, 206, and 423 patients, respectively. All subjects were informed about the procedures, purpose, and study course, and every participant provided his or her signed written consent. The research protocol was approved by the Ethics Committee of the Liaocheng People's Hospital (201803007). This study was conducted in compliance with the principles of the Declaration of Helsinki and its subsequent amendments.
2.2. Inclusion criteria
The diagnostic standards for HCC issued by the National Health Commission of the People's Republic of China were as follows: A) contrast-enhanced ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) results indicating typical imaging lesions of HCC, and the lesion site had typical blood flow changes or B) suspected small nodules found using contrast-enhanced ultrasound, CT, or MRI were confirmed by positron emission tomography examination; C) pathological examination results were consistent with the histopathological diagnosis of HCC. The diagnostic criteria for chronic liver diseases (hepatitis or CH) were as follows: (1) histopathological diagnosis or (2) in the absence of a histological diagnosis, ultrasound, CT, or MRI imaging results indicated splenomegaly without liver space-occupying lesions. The diagnostic criteria for cirrhosis were as follows: (1) the determination of liver fibrosis status was carried out utilizing Metavir scoring system. (2) in the case of non-cirrhotic portal hypertension, endoscopy revealed varices in the esophagogastric or digestive tract that were not in the usual location (3) the characteristics of liver cirrhosis or portal hypertension were evident in B-ultrasound or CT and other imaging examination such as the presence of an irregular liver outline, abnormal proportion of liver lobes, or uneven liver density. Indirect indications of liver cirrhosis encompass esophageal and gastric varices, ascites, and splenomegaly. (4) in patients lacking histological, endoscopic or imaging examinations, the presence of following abnormalities indicates liver cirrhosis, as suggest by 2 out of 4 following items): 1) platelet count (PLT) < 100 × 109/L, with no other evident cause; 2) serum albumin <35 g/L, excluding malnutrition or kidney disease as underlying causes; 3) international normalized ratio (INR) > 1.3 or prolonged prothrombin time (PT) (after discontinuation of thrombolysis or anticoagulants for more than 7 days). 4) AST/PLT ratio index (APRI): adult APRI score >2.
2.3. Exclusion criteria
The patients with the following diseases were excluded: (1) autoimmune liver disease; (2) ICC; (3) drug-induced hepatitis; (4) warfarin or vitamin K treatment; (5) metabolic liver disease; (6) schistosomiasis.
2.4. AFP, AFP-L3, and PIVKA II tests
Blood samples were collected in coagulation tubes and the plasma was separated via centrifugation at 3000 rpm for 10 min. The plasma was then used for further analyses. The biomarkers were measured using the μTASWako i30 automatic immunofluorescence analyzer (FUJIFILM Wako Pure Chemical Corporation, Japanese). The reagent was sourced from the μTASWako i30 Medical Company. The minimum quantitative limit of AFP was 0.5 ng/mL, while that of AFP-L3% was 0.5 %. The minimum quantitative limit of PIVKA II was 5 mAU/mL. In the logistic regression analysis, 0.5 % represented AFP-L3 <0.5 %, 99.5 % represented AFP-L3% >99.5 %, and 5 % represented PIVKA II < 5 mAU/mL.
2.5. Statistical analysis
SPSS version 24.0 software, GraphPad Prism version 5.0, and MedCalc statistical software were employed to perform the analyses. p < 0.05 and < 0.01 were considered to indicate statistical significance. Correlations between HCC and sex were calculated by the Chi-square test. Correlations between HCC and age, TB, AST, ALP, and GGT were analyzed by Mann–Whitney U test. p < 0.05 and <0.01 were considered to indicate statistical significance. The feasibility of using AFP/AFP-L3/PIVKA II as a potential biomarker for distinguishing patients with HCC from CLD was assessed through receiver operating characteristic (ROC) curve analysis. The ROC curve analysis was performed by calculating the maximal area under the curve (AUC). Logistic regression analyses adjusted for age, AFP, AFP-L3, PIVKAII, ALP, AST, GGT, and TB was applied to determine the significant independent predictors of HCC. MedCalc software was used to analyze the diagnostic performance of the respective tests between each diagnostic model.
3. Results
3.1. The demographic and clinical characteristics of patients
In this study, 1241 patients (395 patients with HCC and 846 with CLD) were enrolled and analyzed. The clinical characteristics of the patients are shown in Table 1. The inclusion and exclusion criteria of patients are described in the Materials and Methods section. We found statistically significant differences between HCC and non-HCC groups in the clinical parameters and the patients with HCC exhibited a higher level of AFP (p < 0.001), AFP-L3 (p < 0.001), PIVKA II (p < 0.001), TB (p < 0.001), DB, ALP, GGT, ALT (p < 0.001), and AST (p < 0.001) (Table 1).
Table 1.
Clinical characteristics of the patients included in the study.
| Variables | All patients (N = 1241) |
p-value* | |
|---|---|---|---|
| HCC (N = 395) | CLD (N = 846) | ||
| Age year | 59 ± 0.6 | 51 ± 0.4 | <0.001 |
| Gender male % (N) † | 81.0(320) | 74.3 %(629) | 0.01 |
| ALT U/L | 85.9 ± 8.5 | 45.0 ± 3.4 | <0.001 |
| AST U/L | 110.9 ± 9.0 | 42.0 ± 3.1 | <0.001 |
| Albumin g/L | 33.5 ± 0.3 | 40.5 ± 0.2 | <0.001 |
| Globulin g/L | 33.2 ± 0.4 | 34.9 ± 0.6 | 0.053 |
| TB μmol/l | 54.5 ± 4.6 | 26.0 ± 1.2 | <0.001 |
| DB μmol/l | 32.1 ± 3.8 | 8.8 ± 0.9 | <0.001 |
| ALP IU/L | 145.2 ± 6.8 | 83.1 ± 2.1 | <0.001 |
| GGT IU/L | 125.7 ± 7.3 | 43.2 ± 2.8 | <0.001 |
| AFP ng/ml | 41318.4 ± 14,953 | 43.9 ± 44.1 | <0.001 |
| AFP-L3 % | 40.5 ± 1.6 | 1.6 ± 0.1 | <0.001 |
| PIVKA II mAU/ml | 14638.7 ± 2244.8 | 183.6 ± 83.6 | <0.001 |
| TNM staging system % (N) | |||
| I | 97(24.6) | ||
| II | 62(15.7) | ||
| III | 66(16.7) | ||
| IV | 170(43.0) | ||
HCC, hepatocellular carcinoma, non-HCC, non-hepatocellular carcinoma, TB, Total bilirubin, DB, Direct bilirubin, ALT, glutamic pyruvic transaminase, AST, Glutamic oxaloacetic transaminase, GGT, Glutamyltransferase, ALP, alkaline phosphatase. *, t-test was for independent samples or Mann-Whitney test. †, chi-square test. Categorical data are presented as whole number and percentage, and continuous data are presented as mean ± SD.
3.2. Serum levels of AFP, AFP-L3, and PIVKA II in patients with CLD and HCC
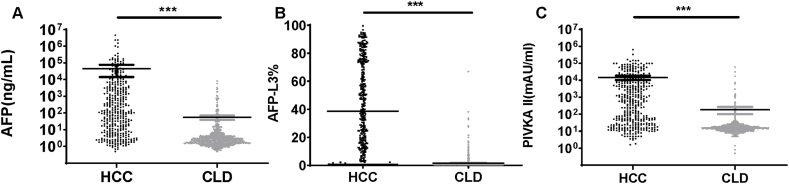
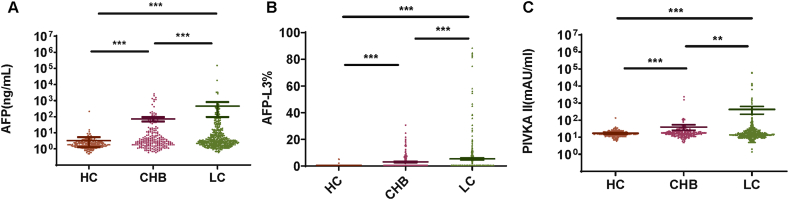
To investigate the diagnosis value of AFP, AFP-L3, and PIVKA II in HCC, we compared the serum levels of AFP, AFP-L3, and PIVKA II in the HCC group and CLD group. The median levels of AFP, AFP-L3, and PIVKA II in the HCC group were significantly higher than those in the CLD group (Fig. 1A–C). Subsequently, the CLD group was divided into the following three groups according to the clinical diagnosis: hepatitis carrier (HC) group, chronic hepatitis B (CHB) group, and liver cirrhosis (LC) group. HC, CHB, and LC groups contained 217, 206, and 423 patients, respectively. We compared the serum levels of AFP, AFP-L3, and PIVKA II in these three subgroups. We found that the serum levels of AFP, AFP-L3, and PIVKA II were significantly different in these three subgroups. The serum levels of AFP, AFP-L3, and PIVKA II in the CHB group were significantly higher than those in the HC group (p < 0.01) (Fig. 2A–C). In the LC group, the serum levels of AFP and AFP-L3 were significantly higher than those in the CHB group (p < 0.01) (Fig. 2A and B). In the LC group, the serum levels of PIVKA II were higher than those in the CHB group (p < 0.05) (Fig. 2C). The serum levels of AFP, AFP-L3, and PIVKA II in the CHB group were significantly higher than those in the HCC group (p < 0.01) (Fig. 2A–C). Increased levels of AFP, AFP-L3, and PIVKA II indicate a high risk of liver disease progression. We also analyzed the association of AFP, AFP-L3, and PIVKA II with various TNM staging in patients with HCC. We observed that high content of AFP, AFP-L3, and PIVKA II were associated with advanced TNM stage (Supplementary Fig. 1). These results indicated that AFP, AFP-L3, and PIVKA II can be potential diagnostic markers for HCC.
Fig. 1.
The serum levels of AFP, AFP-L3, and PIVKAII in the HCC and CLD groups. p < 0.001 ***. (A)The serum levels of AFP in HCC and CLD groups. (B) The serum levels of AFP-L3 in HCC and CLD patients. (C) The serum levels of PIVKAII in HCC and CLD groups.
Fig. 2.
The serum levels of AFP, AFP-L3, and PIVKAII in the subgroups of CLD patients. (A) The serum levels of AFP in the subgroups of CLD groups. (B) The serum levels of AFP-L3 in the subgroups of CLD groups. (C) The serum levels of PIVKAII in the subgroups of CLD groups. Abbreviations: CLD: chronic liver disease, HC: Viral hepatitis carrier, CHB: chronic hepatitis B, LC, liver cirrhosis.
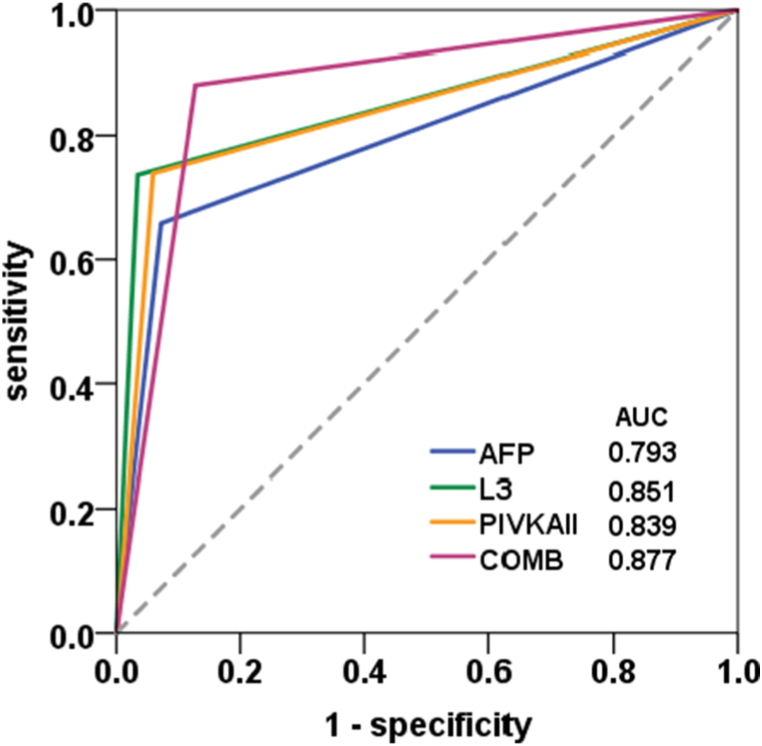
A single marker as AFP has been reported in the diagnosis of HCC; however, its diagnostic value is limited by low specificity and positive predictive value. In this study, we compared the ROC curves based on the cutoff value of 20.0 ng/mL for AFP, AFP-L3 was 10 %, and PIVKAII 40 mAU/mL. The AUC value of AFP, AFP-L3, and PIVKA II was 0.73, 0.851, and 0.839 respectively. Meanwhile, the AUC value of combined biomarkers was 0.877 (Fig. 3). Compared to the limitation of single biomarker detection, the combined diagnosis of HCC markers can increase the accuracy of HCC diagnosis.
Fig. 3.
The ROC curves of AFP, AFP-L3, and PIVKAII and the combined diagnosis. X-axis: Specificity, Y-axis: Sensitivity. The AUC value of AFP, AFP-L3, and PIVKAII and the combined diagnosis were 0.793, 0.851, 0.839, and 0.877, respectively. Abbreviations: L3: AFP-L3, COMB: combined diagnosis.
3.3. The establishment and validation of the AALP model
In this study, we found that AFP, AFP-L3, and PIVKA II were associated with clinical characteristics. To construct a diagnostic model for HCC, we summarized all the independent potential factors affecting the count of diagnostic models by performing binary logistic regression analysis. The independent diagnostic factors of patients with HCC were found and analyzed (Table 2). In this model, age, AFP, AFP-L3, and PIVKAII levels were risk factors for the diagnostic score of HCC. In this model, sex was not a risk factor for the diagnostic score of HCC (p > 0.05) (Supplementary Table 1). Thus, the diagnostic model was constructed based on age, AFP-L3, AFP, and PIVKA II. In this model, AFP-L3 scores were converted to 0 if AFP-L3 > 10 %, otherwise, AFP-L3 scores were converted to 1. The HCC diagnostic score was calculated by the formula as follows: Z = −7.245 + 0.056 × age +0.431 log10 (AFP) + 3.112 × AFP-L3 (0 for AFP-L3 < 10 %, 1 for AFP-L3 ≥ 10 %) + 1.162 × log10 (PIVKA II). Table 3 presents 95 % prediction and confidence intervals. These results showed that the serum levels of AFP, AFP-L3, and PIVKAII and age could be effective risk factors for HCC with an AUC value of 0.939 using this diagnostic model (Fig. 4 and Table 3). In this model, the p-value of risk factors <0.05, and the p-value of the Hosmer–Lemeshow test was 0.782 (>0.05). Therefore, the predictive performance of the established model can be accepted.
Table 2.
Significant independent factors affecting the diagnostic values in patients with HCC due to binary logistic regression analysis.
| Multivariate analysis for Z score |
||||
|---|---|---|---|---|
| β | SE | 95%CI | p value | |
| Age | 0.056 | 0.009 | 1.039–1.076 | 0.000 |
| Log PIVKAII | 1.162 | 0.135 | 2.453–4.166 | 0.000 |
| Log AFP | 0.43 | 0.131 | 1.19–1.988 | 0.001 |
| AFP-L3 | 3.112 | 0.285 | 12.854–39.247 | 0.000 |
The p value of Hosmer-Lemeshow test was 0.782.
95%CI: 95 % Confidence Interval, SE: standard error, β: standardized coefficients.
Table 3.
Diagnostic performance of the GALAD, GALAD-C, GAAP, and AALP model for HCC.
| Model | AUC (95%CI) | p value | Standard error |
|---|---|---|---|
| AALP | 0.939(0.923–0.955) | 0.000 | 0.008 |
| AFP | 0.793(0.763–0.823) | 0.000 | 0.015 |
| AFP-L3 | 0.851(0.824–0.867) | 0.000 | 0.014 |
| PIVKA II | 0.839(0.854–0.899) | 0.000 | 0.014 |
| Combined | 0.877(0.854–0.899) | 0.000 | 0.012 |
| GALAD | 0.930(0.913–0.947) | 0.000 | 0.009 |
| GALAD-C | 0.937(0.920–0.953) | 0.000 | 0.008 |
| GAAP | 0.916(0.897–0.934) | 0.000 | 0.010 |
95%CI: 95 % Confidence Interval, AUC: area under the ROC curve, combined: combined AFP, AFP-L3 and PIVKA II.
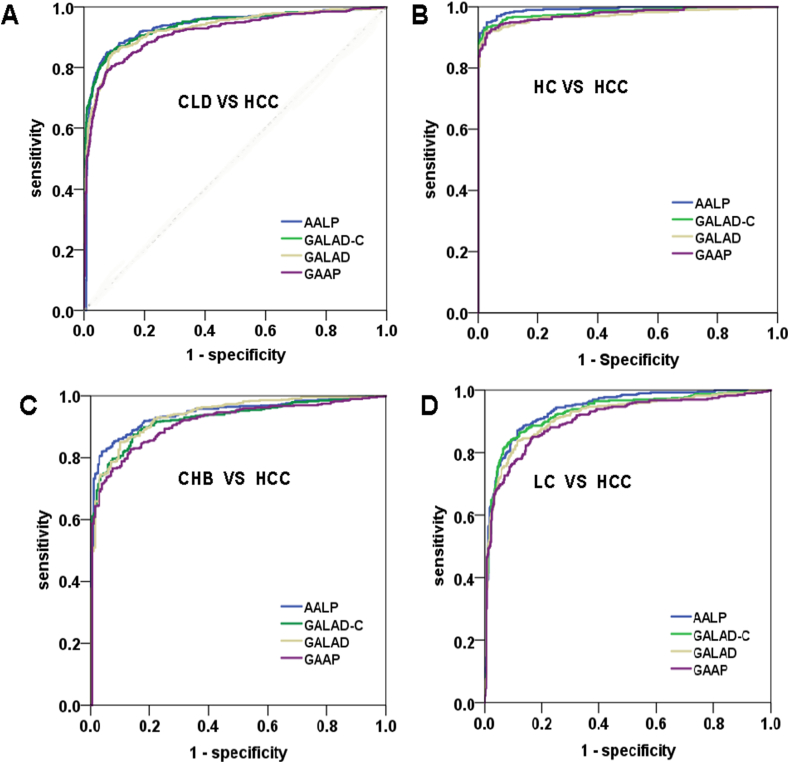
Fig. 4.
Comparisons of GALAD, GALAD-C, GAAP, and AALP models. (A) The ROC curves for diagnosis of HCC by GALAD, GALAD-C, GAAP, and ALAP models. (B) The ROC curves for diagnosis of HCC in the four models compared HCC patients with the HC group. (C) The ROC curves for diagnosis of HCC in these four models compared HCC patients with the CHB group. (D) The ROC curves for the diagnosis of HCC in the four models compared HCC patients with the LC group. X-axis: Specificity, Y-axis: Sensitivity. CLD: chronic liver disease, HC: Viral hepatitis carrier, CHB: chronic hepatitis B, LC, liver cirrhosis.
3.4. Comparison of GALAD, GALAD-C, GAAP, and AALP model
The GALAD model was described using the following equation [18]:
Z = −10.08 + 0.09 × age+1.67 × sex+2.34log10 (AFP) +0.04 × AFP-L3+1.33 × log10 (PIVKA II), sex = 1 for males and = 0 for females.
The GALAD-C model was described using the following equation [19]:
Z = −11.501 + 0.099 × age+0.073 × sex+0.84 log10 (AFP) +0.073 × AFP-L3+2.364 × log10 (PIVKA II), sex = 1 for males and = 0 for females.
The GAAP model was described using the following equation [19]:
Z = −11.203 + 0.094 × age+0.699 × sex+1.076 log10 (AFP) +2.376 × log10 (PIVKA II), sex = 1 for males and = 0 for females.
The AALP model was described using the following equation:
Z = −7.245 + 0.056 × age+0.431 log10(AFP)+3.112 × AFP-L3(0 for AFP-L3<10 %, 1 for AFP-L3≥10 %) +1.162 × log10(PIVKA II).
The probability of HCC (P (HCC)) in an individual patient was calculated using the following formula: P (HCC) = exp (Z)/(1+ exp (Z))
The GALAD, GALAD-C, GAAP, and AALP models showed good prognostic value for patients with HBV/HCV having HCC (Fig. 4A). The GALAD, GALAD-C, GAAP, and AALP models also showed good prognostic value for subgroups of CLD patients (Fig. 4B–D). In the GAAP model, the AUC of the ROC curves was 0.916 (95 % CI = 0.897–0.934, p < 0.001). In the GALAD model, the score was 0.930 (95 % CI = 0.913–0.947, p < 0.001). In the AALP model, the score was 0.937 (95 % CI = 0.920–0.953, p < 0.001). In the GALAD-C model, the score was 0.939 (95 % CI = 0.923–0.955, p < 0.001) (Table 3 and Fig. 4A). Furthermore, we used MedCalc software to compare these four models and found that the AALP model was superior to the GALAD-C model and GAAP model (p < 0.01). GALAD-C model was superior to GALAD model (p < 0.01). The AALP model was not superior to the GALAD model (p > 0.05) (Supplementary Table 2). The sensitivity and specificity of the AALP model were 0.85 and 0.923 respectively. The difference between the GALAD-C model, GALAD model, and GAAP model was very less in terms of sensitivity and specificity (Supplementary Table 3). These results indicated that the AALP model exhibited good sensitivity for predicting the likelihood of HCC.
3.5. The diagnostic value and monitoring recurrence in patients with HCC
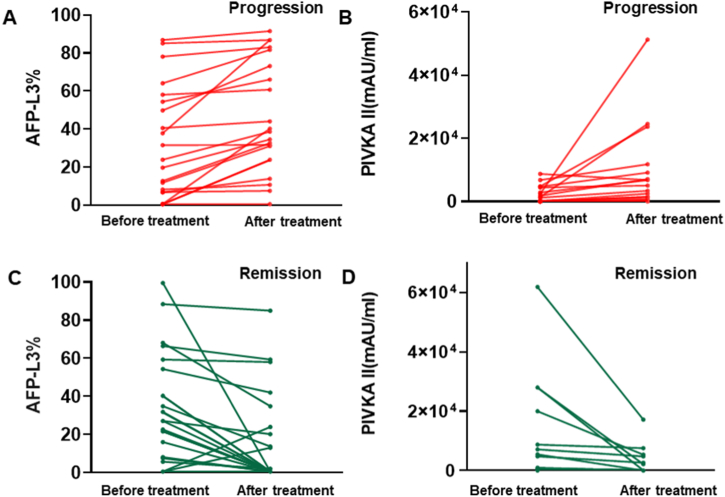
The above studies showed that AFP-L3 and PIVKA II serum levels were better predictors of HCC diagnosis than the AFP serum level. Nevertheless, potential of AFP-L3 and PIVKA II for postoperative recurrence diagnosis has not been systematically studied yet. HCC recurrence is often detected by imaging examinations [19]. To assess AFP-L3 and PIVKA II levels to detect HCC postoperative recurrence, we included patients with HCC who underwent various treatment, included ablation, TACE and resection. First, we compared AFP-L3 and PIVKA II serum levels in patients with HCC showing progression. In most of the patients, either serum levels of AFP-L3 or PIVKA II increased according to the line charts (Fig. 5A and B). The AFP-L3 value increased from 0.5 to 40.2 maximally. The PIVKA II value increased from 2480 to 51,302 maximally (Supplementary Table 4). Some exceptional changes were observed in patients showing HCC progression, and AFP-L3 and PIVKA II serum levels did not increase in these patients.
Fig. 5.
The dynamic changing of AFP-L3 and PIVKA II before and after receiving treatment in HCC patients with progression (A and B) and remission (C and D). The content patterns are presented by line chart according to AFP-L3 and PIVKA II values.
Similarly, we compared AFP-L3 and PIVKA II serum levels in patients with HCC remission. Either AFP-L3 or PIVKA II serum levels decreased in a majority of patients with HCC remission (Fig. 5C and D). The AFP-L3 value decreased from 99.5 to 0.5 maximally. The PIVKA II value decreased from 61,831 to 17,167 maximally. However, there were some exceptions. The AFP-L3 value increased from 0.5 to 13 in one patient with HCC who showed no progression, whereas the PIVKA II value increased from 13 to 178 in another patient showing no progression (Supplementary Table 5).
To verify whether AFP, AFP-L3, and PIVKA II levels could be used for monitoring dynamic disease statuses, we further selected four patients with HCC and tracked serum levels of these three biomarkers before and after diagnosis or treatment (Table 4). The imaging results of the patients (CT or MRI) were systematically analyzed. Then, we generated a timeline to assess the consistency between the imaging results and the serological results. The positive serological test criteria for these three biomarkers were AFP >20 ng/mL, AFP-L3 > 10 %, and PIVKA II > 40 mAU/mL.
Table 4.
The serum levels of AFP, AFP-L3, and PIVKA II at different time points throughout the treatment course. The positive serological test criteria of these three biomarkers were AFP >20 ng/mL, AFP-L3 >10 %, and PIVKA II > 40 mAU/mL, respectively.
| Date | AFP (ng/mL) | AFP-L3(%) | PIVKA II (mAU/mL) | Disease status | |
|---|---|---|---|---|---|
| Case 1 |
2020.11 | 127.9 | 82.8 | 294 | HCC, multiple lesions |
| 2021.2 | 18.8 | 71.3 | 30 | Single lesions Tumor size 10.5 × 9.5 cm |
|
| 2021.8 |
3.1 |
<0.5 |
6 |
Remission |
|
| Case 2 |
2021.1 | 18.5 | 86.9 | 743 | HCC Tumor size 3.5 × 3.1 cm |
| 2021.2 | 32.6 | 85.1 | 212 | HCC Tumor size 3.2 × 3.0 cm |
|
| 2021.4 | 128 | 91.5 | 1402 | HCC Tumor size 3.5 × 4.0 cm |
|
| 2021.5 |
465.5 |
91.8 |
24,565 |
HCC Tumor size 4.9 × 6.1 cm Portal vein tumor thrombus |
|
| Case 3 |
2020.3 | 6.1 | <0.5 | 13 | liver cirrhosis |
| 2020.12 | 7.9 | 38.6 | 15 | HCC, single lesions Tumor size 2.1 × 2.1 × 1.7 cm |
|
| 2021.4 |
3.5 |
<0.5 |
27 |
Remission |
|
| Case 4 | 2019.1 | 6.7 | 9.1 | 2480 | HCC, multiple lesions |
| 2019.12 | 8.2 | 8.4 | 6801 | progression |
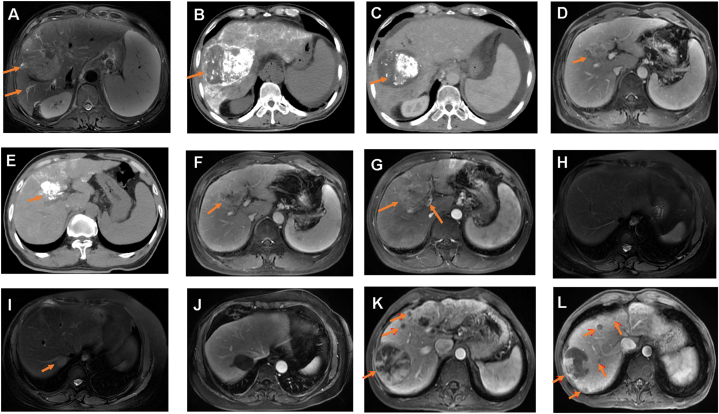
Case 1: Age, 58; Male. The person was diagnosed with HCC in November 2020 and multiple lesions were found (Fig. 6A). AFP-L3 and PIVKA II test results were positive at this point (Table 4). Subsequently, the patient received transhepatic arterial chemotherapy and embolization (TACE). Only one lesion was observed and the patient was not in remission according to CT (Fig. 6B). However, remission was observed in August 2021 (Fig. 6C). AFP-L3 and PIVKA II serological test results were negative at this point. During this process, serum AFP, AFP-L3, and PIVKA II levels decreased gradually. AFP-L3 levels decreased from 82.8 % to 71.3 % and from 71.3 % to <0.5 %. PIVKA II levels decreased from 294 mAU/mL to 30 mAU/mL and from 30 mAU/mL to 6 mAU/mL (Table 4). AFP levels decreased from 127.9 ng/mL to 18.8 ng/mL and from 18.8 ng/mL to 3.1 ng/mL.
Fig. 6.
Dynamic imaging results from 4 clinical cases. (A–C) Case 1, this case became remission. (D–G) Case 2, this case progressed with enlarged tumor. (H–J) Case 3, this case progressed from liver cirrhosis to HCC and finally became remission. (K–L) Case 4, this case progressed with new lesions. (A)Case 1, initial imaging results with elevated AFP, AFP-L3, and PIVKA II. MRI showing two lesions of HCC (arrows). (B) Case 1, CT showing a tumor with parenchymal iodized oil deposition (tumor size 10.5 × 9.5 cm) (arrow). (C) Case 1, CT showing an inactive lesion. (D) Case 2, MRI showing an active lesion, tumor size 3.5 × 3.1 cm (arrow). (E) Case 2, MRI showing this lesion size becoming 3.2 × 3.0 cm after radiofrequency therapy. (F) Case 2, a larger lesion size was observed (arrow), with tumor size 3.5 × 4.0 cm. (G) Case 2, this pre-present lesion became larger (4.9 × 6.1 cm) and PVTT appeared. (H) Case 3, MRI showing no HCC detection in this case. (I) Case 3, this case was diagnosed as HCC with a single lesion (arrow). (J) Case 3, No active lesion was observed and this case went into remission. (K) Case 4, multiple lesions were located in the right liver lobe (arrows). (L) Case 4, a new lesion was detected in the right liver lobe relative to that before MRI imaging (arrows).
PVTT, portal vein tumor thrombus.
Case 2: Age, 53; Male. The person initially experienced recurrence after surgical treatment in January 2021 with positive AFP-L3 and PIVKA II results (86.9 % and 743 mAU/mL, respectively) (Table 4). However, liver cancer had progressed according to imaging results. The tumor size was 3.5 × 3.1 cm (Fig. 6D). In February 2021, the serological test results were 85.1 % and 212 mAU/mL with a tumor size of 3.2 × 3.0 cm (Fig. 6E). In April 2021, the serological results were 91.5 % and 1402 mAU/mL with a tumor size of 3.5 × 4.0 cm. In May 2021, the serological test results were 91.8 % and 24,565 mAU/mL with a tumor size of 4.9 × 6.1 cm (Fig. 6F). Moreover, portal vein tumor thrombus (PVTT) was observed (Fig. 6G). Hence, AFP, AFP-L3, and PIVKA II levels increased as time progressed with an increased size of the lesion (Table 4).
Case 3: Age, 60; Female. The person was initially diagnosed with liver cirrhosis and showed negative results for AFP, AFP-L3, and PIVKA II in June 2020 (Fig. 6H and Table 4). The disease progressed into liver cancer with AFP-L3-positive and PIVKA II-negative results in September 2020(Fig. 6I). After receiving radiofrequency ablation, cancer remission was observed and the serum test turned negative (Fig. 6J). AFP-L3 serum levels increased significantly from <0.5 % to 38.6 %, whereas PIVKA II serum levels were less than 40 mAU/mL, showing a negative result in clinical testing.
Case 4: Age, 48; Male. The person presented multiple lesions after surgical resection (Fig. 6K). The serological results were PIVKA II-positive (2480 mAU/mL) and AFP-L3-negative (9.1, <10 %) in January 2019 (Table 4). Later, MRI results showed enlarged multiple lesions as well as new lesions in December 2019. This case had progression of disease (Fig. 6L). The serological results showed were PIVKA II-positive (6801 mAU/mL) and AFP-L3-negative (8.4 %) at this time.
Based on the above comparison and analyses, we found that these two independent serum biomarkers showed similar performances and were complementary to the diagnosis and monitoring of HCC. Thus, AFP-L3 and PIVKA II can be considered potential prognostic biomarkers.
4. Discussion
The majority of HCC arises from liver cirrhosis and chronic liver diseases (CLDs) [20]. The common risk factors for HCC included viral hepatitis (HBV/HCV), alcoholic abuse, and non-alcoholic fatty liver disease [5,21]. HCC diagnosis and staging are predominantly based on pathological and imaging studies. HCC should be screened routinely via abdominal ultrasound and evaluation of serum alpha-fetoprotein (AFP) levels every 6 months [22]. However, pathological analysis is invasive and has certain disadvantages, such as obtaining adequate tissues is difficult and surgical risks may exist. Serological testing has the advantages of being non-invasive and allowing real-time monitoring; thus, it can be a potential alternative to pathological testing. AFP is used to diagnose, monitor, and terminate HCC; however, it has limited sensitivity and specificity. To improve the prognosis of patients with HCC, identifying more effective biomarkers is essential. A systematic review suggested that AFP and AFP-L3 combined with PIVKA II could potentially be a valuable prognosis tool for patients with HCC [23].
Here, we evaluated the diagnostic value of AFP-L3 and PIVKA II levels in patients with HCC. The diagnostic value of serum AFP-L3 and PIVKA II levels was found to be better than that of serum AFP levels. Moreover, we showed that the AUC value of the combination of AFP, AFP-L3, and PIVKA II was 0.877, the sensitivity was 81.0, and the specificity was 95.5. The combined diagnosis of the three markers greatly improved the early diagnosis accuracy of HCC. This finding is consistent with other study findings [[24], [25], [26]]. AFP combined with AFP-L3 and PIVKA II can improve the sensitivity of HCC diagnosis, especially for patients with AFP-negative HCC.
To improve the diagnostic accuracy, an HCC prediction model, AALP, was established based on age and the three markers. In the binary logistic regression model, these three biomarkers were significantly related to patients with HCC. The identified signature was integrated with clinical features to establish a composite diagnostic model, which reliably demonstrated accurate diagnostic predictions for patients with CLD and HCC. The AUC value of the AALP model was 0.939 (95 % CI: 0.923–0.955), which indicated that AALP model performed good in predicting HCC accurately. The AALP model also showed good performance in differentiating patients with HCC from subgroups of patients with CLD.
In the AALP model, age and AFP, AFP-L3, and PIVKA II levels were risk factors and these results were consistent with previous published findings [19]. For example, the incidence of HCC increases with age, and older age is independently associated with HCC development [11,27,28]. AFP-L3 has been suggested as a biomarker for early HCC detection due to its higher specificity than AFP [11]. PIVKA II exhibits higher specificity and sensitivity than AFP for HCC diagnostic [22]. Furthermore, increased AFP, AFP-L3, and PIVKA II serum levels have been observed in patients with HCC than in patients with CLD [11,13,29].
However, in this model, sex was not a risk factor unlike it was in the other three models. This finding is inconsistent with previous findings [11,18]. There may be several possible reasons for this result. One possible reason is differences in populations enrolled in the studies. Another possible reason is the different methodological approaches used in the studies. Herein, binary logistic regression was performed using the stepwise backward method. Furthermore, unlike other models, AFP-L3 was calculated as 0 or 1 in the AALP model. These reasons may explain why sex was not a risk factor in the present model.
To validate the performance of the AALP model, we compared it with the other three models. Liu et al. built the GALAD-C model for Chinese patients with HCC and validated that GALAD-C showed better performance than the GALAD model built for patients from the USA [19]. This finding is consistent with our findings and the hypothesis that enrolling different populations results in different diagnostic models has been confirmed. Another study also revealed that differences in demographic and biochemical characteristics led to different results. Further scientific studies based on a multicenter clinical trial are required to verify the present findings.
Further, to validate the performance of the combination of AFP, AFP-L3, and PIVKA II for diagnosing and monitoring patients with HCC, we collected the clinical samples and disease statuses of the patients. By comparing the serological results with the imaging results, we subsequently validated the diagnostic capabilities of the combination of AFP, AFP-L3, and PIVKA II for detecting and monitoring HCC. HCC always develops in response to chronic inflammation of the liver, and AFP levels are closely related to tumor size, stage, and pathological grade [30]. Patients undergoing surgical resection with recurrence often present increased AFP levels [31]. Similar results could be found for AFP-L3 and PIVKAII [32,33]. Decreased AFP-L3 and/or PIVKA II levels generally indicate a good prognosis, whereas increased AFP-L3 and/or PIVKA II levels suggest a poor prognosis [[34], [35], [36]]. Here, patients with HCC were categorized into three subgroups: increased AFP-L3 and PIVKA II levels, increased AFP-L3 levels, and increased PIVKA II levels. In Case 1 and Case 2, when tumors were present, or tumor recurrence occurred, AFP-L3 and PIVKA II tests were positive. In Case 3, when the tumor was observed by imaging results, AFP-L3 was positive and PIVKA II was negative. After treatment, remission was observed in this patient with AFP-L3-negative results. In Case 4, when local tumor progression was observed, the serum AFP-L3 test was negative and PIVKA II was positive. AFP-L3 and PIVKA II levels can predict CLD progressing to HCC. These cases also showed the utility of AFP-L3 and PIVKA II levels in monitoring recurrence in patients with HCC who were clinically treated. Previous studies showed that the correlation between AFP-L3 and PIVKA II levels was low and they were two independent biomarkers [37,38]. AFP-L3 is particularly useful in predicting HCC recurrence after receiving radiofrequency ablation [39]. PIVKA II is particularly useful in predicting HCC with PVTT [40]. AFP-L3 and PIVKA II are useful in patients with low levels of AFP (<20 ng/mL). Overall, the present study shows that two biomarkers, AFP-L3 and PIVKA II, complement each other and are not irreplaceable. Further, both these biomarkers are recommended to use in clinical applications. Nevertheless, several limitations should be noted. First, this research was a single center research. further this new model should be validated in multicenter studies. Second, we were unable to analyze combined biomarkers according to pathological stage.
5. Conclusion
In conclusion, we developed and validated the AALP model to predict CLD in patients with HCC. The proposed model yielded statistically significant results, which were better than those yielded by the current GALAD-C model. The present results suggest that AFP combined with AFP-L3 and PIVKA II can be used as a prognosis biomarker and can increase the accuracy of HCC diagnosis. Moreover, AFP-L3 and PIVKA II levels are complementary to each other but irreplaceable. These results provide a valuable theoretical basis for the utility of AFP-L3 and PIVKA II levels for diagnosing HCC and monitoring its recurrence.
Data availability statement
Data will be made available on request.
Funding
This research was funded by Natural Science Foundation of Shandong Province (No. ZR2021MH215),Liaocheng Key R&D Plan (2022YDSF37), Medical and Health Technology Development Plan Project of Shandong Province (202204080720) and Scientific Research Fund of Liaocheng People's Hospital (LYQN201935).
CRediT authorship contribution statement
Tianying Ren: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Data curation. Xu Hou: Software, Methodology, Formal analysis, Data curation. Xin Zhang: Software, Methodology, Investigation, Formal analysis, Data curation. Dongliang Chen: Visualization, Methodology, Data curation. Juan Li: Validation, Data curation. Yingnan Zhu: Software, Formal analysis. Zhiheng Liu: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Investigation, Conceptualization. Dawei Yang: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21906.
Contributor Information
Zhiheng Liu, Email: lzh8623@163.com.
Dawei Yang, Email: yangdawei775@163.com.
Abbreviations
- AFP
alpha-fetoprotein
- AFP-L3
lens culinaris agglutinin-reactive fraction of AFP
- PIVKA II
protein induced by vitamin K absence or antagonist-II
- CLD
chronic liver disease
- HCC
hepatocellular carcinoma
- ROC
receiver operating characteristics
- AUC
area under the curve
- CI
confidence interval
- CT
computed tomography
- MRI
magnetic resonance imaging
- PVTT
portal vein tumor thrombus
- TACE
transcatheter arterial chemoembolization and embolization
- RFA
received radiofrequency ablation
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Xia C., Dong X., Li H., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng R., Su Q., Huang X., et al. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun. 2023;43(1):75–86. doi: 10.1002/cac2.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Fang M., Xiao X., et al. Validation of the galad model for early diagnosis and monitoring of hepatocellular carcinoma in Chinese multicenter study. Liver Int. 2022;42(1):210–223. doi: 10.1111/liv.15082. [DOI] [PubMed] [Google Scholar]
- 4.Liu S.Y., Li C., Sun L.Y., et al. Asap score versus galad score for detection of hepatitis c-related hepatocellular carcinoma: a multicenter case-control analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumgay H., Arnold M., Ferlay J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyoda H., Kumada T., Tada T., et al. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J. Hepatol. 2012;57(6):1251–1257. doi: 10.1016/j.jhep.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Du Z., Liu X., Wei X., et al. Quantitative proteomics identifies a plasma multi-protein model for detection of hepatocellular carcinoma. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-72510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X.J., Shao D.H., He M.L., et al. Association of common variants in hnf1a gene with serum afp level in healthy Chinese individuals and hcc patients. Dis Markers. 2019. 2019 doi: 10.1155/2019/6273497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai D.-S., Zhang C., Chen P., et al. The prognostic correlation of afp level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-12834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caviglia G.P., Ciruolo M., Abate M.L., et al. Alpha-fetoprotein, protein induced by vitamin k absence or antagonist ii and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers. 2020;12(11) doi: 10.3390/cancers12113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D., Mallory T., Satomura S. Afp-l3: a new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta. 2001;313(1):15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 13.Choi J.Y., Jung S.W., Kim H.Y., et al. Diagnostic value of afp-l3 and pivka-ii in hepatocellular carcinoma according to total-afp. World J. Gastroenterol. 2013;19(3):339–346. doi: 10.3748/wjg.v19.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga H., Iwamoto H., Suzuki H., et al. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Japanese perspective. Clin. Mol. Hepatol. 2023;29(2):242–251. doi: 10.3350/cmh.2023.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok A.S., Sterling R.K., Everhart J.E., et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svobodova S., Karlikova M., Topolcan O., et al. Pivka-ii as a potential new biomarker for hepatocellular carcinoma - a pilot study. In Vivo. 2018;32(6):1551–1554. doi: 10.21873/invivo.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S., Sun L., Yao L., et al. Diagnostic performance of AFP, AFP-L3, or PIVKA II for hepatitis c virus-associated hepatocellular carcinoma: a multicenter analysis. J. Clin. Med. 2022;11(17) doi: 10.3390/jcm11175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J.D., Addissie B.D., Mara K.C., et al. Galad score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of galadus score. Cancer Epidemiol. Biomarkers Prev. 2019;28(3):531–538. doi: 10.1158/1055-9965.EPI-18-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M., Wu R., Liu X., et al. Validation of the galad model and establishment of gaap model for diagnosis of hepatocellular carcinoma in Chinese patients. J. Hepatocell. Carcinoma. 2020;7:219–232. doi: 10.2147/JHC.S271790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omata M., Cheng A.L., Kokudo N., et al. Asia-pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan M.C., Ouyang W., Liu S.Y., et al. Alpha-fetoprotein, protein induced by vitamin k absence or antagonist-ii, lens culinaris agglutinin-reactive fraction of alpha-fetoprotein alone and in combination for early detection of hepatocellular carcinoma from nonalcoholic fatty liver disease: a multicenter analysis. Hepatobiliary Pancreat. Dis. Int. 2022;21(6):559–568. doi: 10.1016/j.hbpd.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Song P., Tang Q., Feng X., et al. Biomarkers: evaluation of clinical utility in surveillance and early diagnosis for hepatocellular carcinoma. Scand. J. Clin. Lab. Invest. Suppl. 2016;245:S70–S76. doi: 10.1080/00365513.2016.1210328. [DOI] [PubMed] [Google Scholar]
- 23.Sato M., Morimoto K., Kajihara S., et al. Machine-learning approach for the development of a novel predictive model for the diagnosis of hepatocellular carcinoma. Sci. Rep. 2019;9(1):7704. doi: 10.1038/s41598-019-44022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Serag H.B., Kanwal F., Davila J.A., et al. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis c and cirrhosis. Gastroenterology. 2014;146(5):1249–1255 e1241. doi: 10.1053/j.gastro.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J., Li Y., Li Z., et al. Clinical utility of decarboxylation prothrombin combined with alpha-fetoprotein for diagnosing primary hepatocellular carcinoma. Biosci. Rep. 2018;38(5) doi: 10.1042/BSR20180044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S.J., Jang J.Y., Jeong S.W., et al. Usefulness of afp, afp-l3, and pivka-ii, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltim.) 2017;96(11) doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi S.W., Choi J.S., Yi J.J., et al. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in korea. Cancer. 2018;124(13):2748–2757. doi: 10.1002/cncr.31406. [DOI] [PubMed] [Google Scholar]
- 28.Asahina Y., Tsuchiya K., Tamaki N., et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis c virus infection. Hepatology. 2010;52(2):518–527. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 29.Lee H.A., Lee Y.R., Lee Y.S., et al. Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein improves diagnostic accuracy for hepatocellular carcinoma. World J. Gastroenterol. 2021;27(28):4687–4696. doi: 10.3748/wjg.v27.i28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C., Xiao G.Q., Yan L.N., et al. Value of alpha-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J. Gastroenterol. 2013;19(11):1811–1819. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh C.N., Chen M.F. Resection of peritoneal implantation of hepatocellular carcinoma after hepatic resection: risk factors and prognostic analysis. World J. Surg. 2004;28(4):382–386. doi: 10.1007/s00268-003-7319-7. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura S., Nouso K., Sakaguchi K., et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am. J. Gastroenterol. 2006;101(9):2038–2043. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee H.W., Song G.W., Lee S.G., et al. Patient selection by tumor markers in liver transplantation for advanced hepatocellular carcinoma. Liver Transplant. 2018;24(9):1243–1251. doi: 10.1002/lt.25056. [DOI] [PubMed] [Google Scholar]
- 34.Kim D.Y., Toan B.N., Tan C.K., et al. Utility of combining pivka-ii and afp in the surveillance and monitoring of hepatocellular carcinoma in the asia-pacific region. Clin. Mol. Hepatol. 2023;29(2):277–292. doi: 10.3350/cmh.2022.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng J., Wang W., Zhang Y., et al. Prognostic role of pre-treatment serum afp-l3% in hepatocellular carcinoma: systematic review and meta-analysis. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouso K., Kobayashi Y., Nakamura S., et al. Prognostic importance of fucosylated alpha-fetoprotein in hepatocellular carcinoma patients with low alpha-fetoprotein. J. Gastroenterol. Hepatol. 2011;26(7):1195–1200. doi: 10.1111/j.1440-1746.2011.06720.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahn K.S., O'Brien D.R., Kim Y.H., et al. Associations of serum tumor biomarkers with integrated genomic and clinical characteristics of hepatocellular carcinoma. Liver Cancer. 2021;10(6):593–605. doi: 10.1159/000516957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou D., Hu G.F., Gao W.C., et al. Hepatocellular carcinoma with tumor thrombus in bile duct: a proposal of new classification according to resectability of primary lesion. World J. Gastroenterol. 2020;26(44):7005–7021. doi: 10.3748/wjg.v26.i44.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Force M., Park G., Chalikonda D., et al. Alpha-fetoprotein (afp) and afp-l3 is most useful in detection of recurrence of hepatocellular carcinoma in patients after tumor ablation and with low afp level. Viruses. 2022;14(4) doi: 10.3390/v14040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T., Yu Y., Liu J., et al. Pivka-ii level is correlated to development of portal vein tumor thrombus in patients with hbv-related hepatocellular carcinoma. Infect. Agents Cancer. 2019;14:13. doi: 10.1186/s13027-019-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.