Abstract
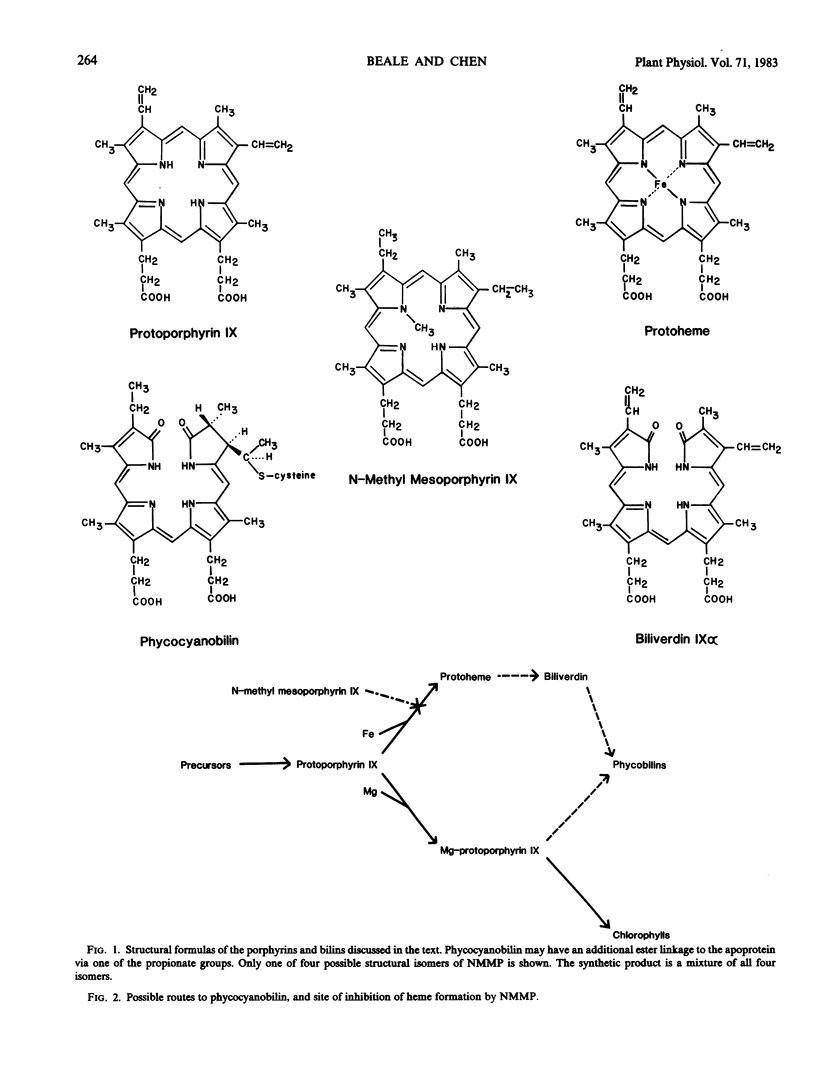
The ability of N-methyl mesoporphyrin IX (NMMP) to block heme synthesis by specifically inhibiting enzymic iron insertion into protoporphyrin IX was exploited to test whether heme is a precursor of the bilin chromophore of phycocyanin (PC). A strain of the unicellular rhodophyte Cyanidium caldarium which forms normal amounts of both chlorophyll (Chl) and PC in the dark was employed to avoid phototoxic effects of exogenous porphyrins. Relative Chl and PC content were assayed spectrophotometrically on whole cell suspensions.
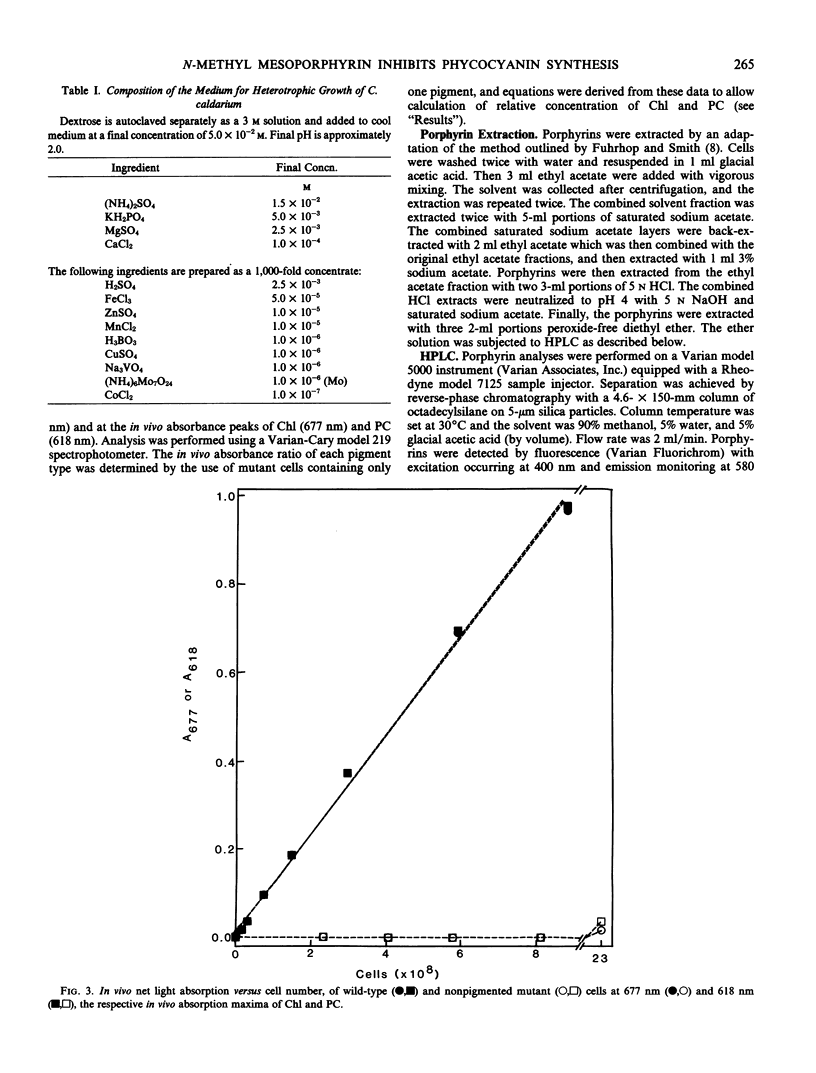
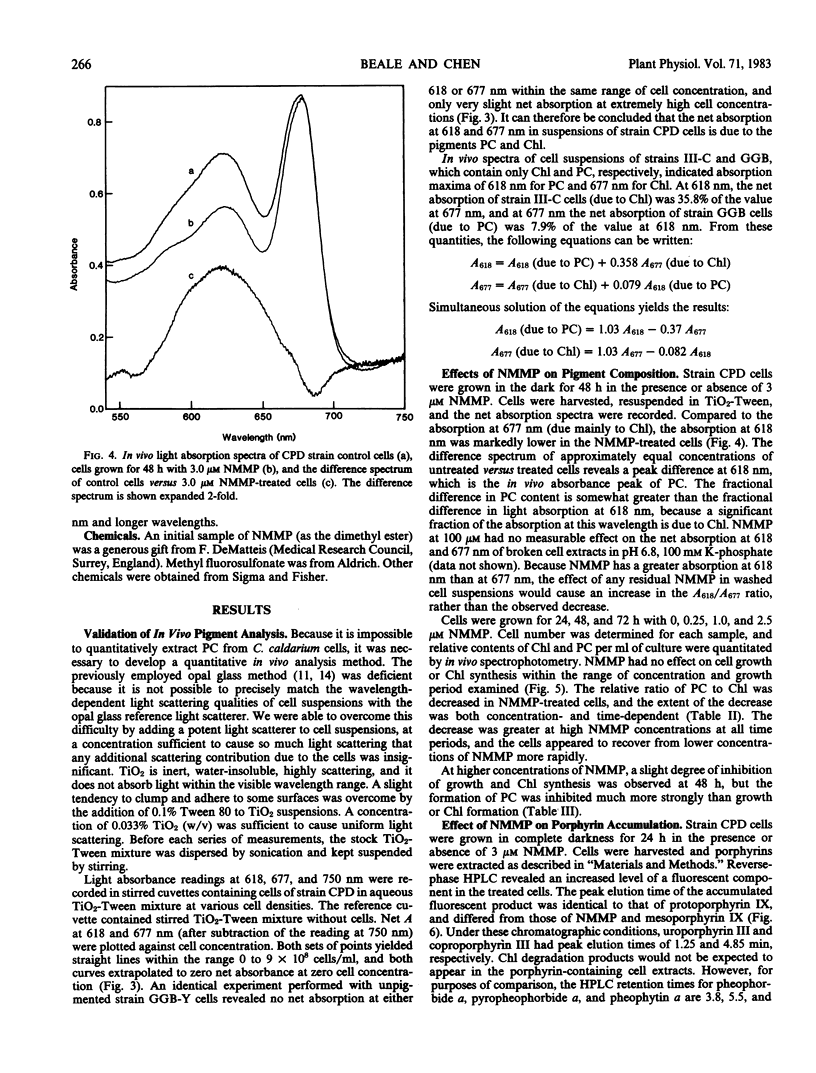
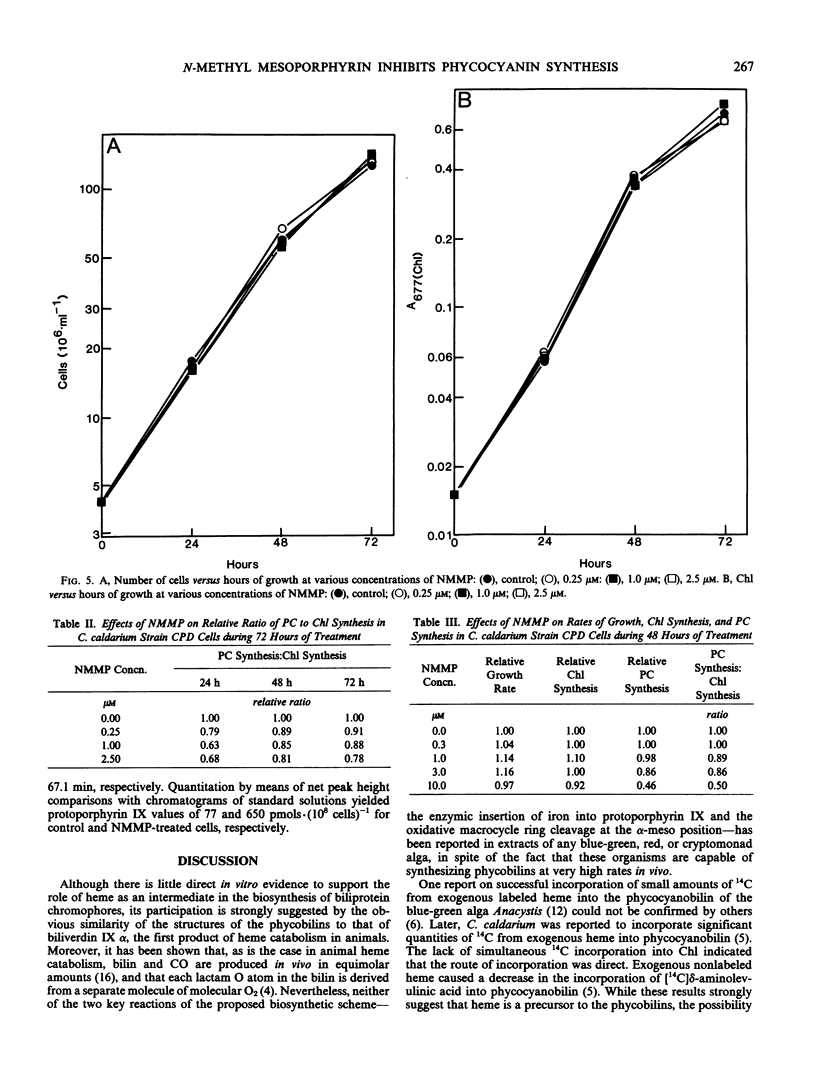
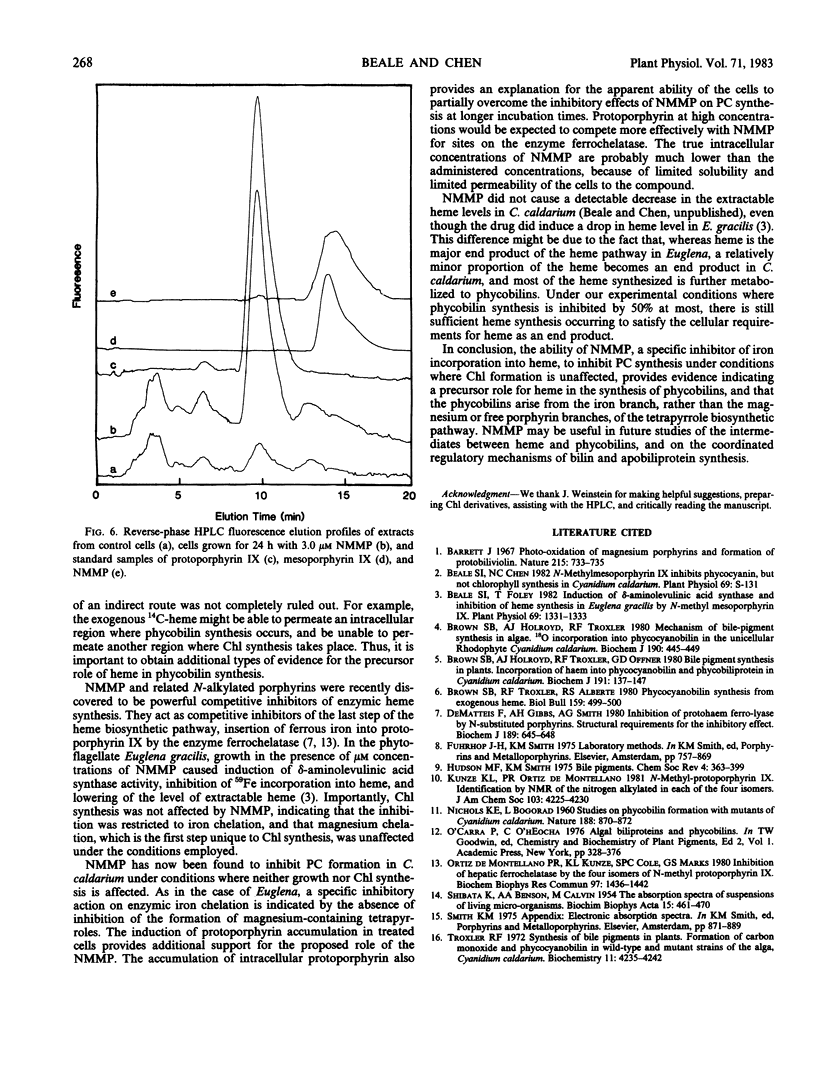
When cells were grown in the dark on a glucose-based heterotrophic medium at 42°C, neither division rate nor Chl synthesis was affected by NMMP up to 3.0 micromolar and for as long as 72 hours. NMMP had a dose-dependent inhibitory effect on PC synthesis. PC to Chl absorbance ratios, relative to control cell values, were 100%, 89%, 86%, and 50% in cells grown for 48 hours with 0.3, 1.0, 3.0, and 10.0 micromolar NMMP, respectively. NMMP also caused the accumulation of intracellular protoporphyrin.
The ability of NMMP to cause intracellular accumulation of protoporphyrin and to block PC synthesis specifically while allowing normal Chl formation is consistent with its action as a specific inhibitor of enzymic iron chelation, and supports the role of heme as a precursor to the phycobilins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Foley T. Induction of delta-Aminolevulinic Acid Synthase Activity and Inhibition of Heme Synthesis in Euglena gracilis by N-Methyl Mesoporphyrin IX. Plant Physiol. 1982 Jun;69(6):1331–1333. doi: 10.1104/pp.69.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Holroyd A. J., Troxler R. F. Mechanism of bile-pigment synthesis in algae. 18O incorporation into phycocyanobilin in the unicellular rhodophyte, Cyanidium caldarium. Biochem J. 1980 Aug 15;190(2):445–449. doi: 10.1042/bj1900445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Smith A. G. Inhibition of protohaem ferro-lyase by N-substituted porphyrins. Structural requirements for the inhibitory effect. Biochem J. 1980 Sep 1;189(3):645–648. doi: 10.1042/bj1890645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS K. E., BOGORAD L. Studies on phycobilin formation with mutants of Cyanidium caldarium. Nature. 1960 Dec 3;188:870–872. doi: 10.1038/188870b0. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Cole S. P., Marks G. S. Inhibition of hepatic ferrochelatase by the four isomers of N-methylprotoporphyrin IX. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1436–1442. doi: 10.1016/s0006-291x(80)80026-x. [DOI] [PubMed] [Google Scholar]
- SHIBATA K., BENSON A. A., CALVIN M. The absorption spectra of suspensions of living micro-organisms. Biochim Biophys Acta. 1954 Dec;15(4):461–470. doi: 10.1016/0006-3002(54)90002-5. [DOI] [PubMed] [Google Scholar]
- Troxler R. F. Synthesis of bile pigments in plants. Formation of carbon monoxide and phycocyanobilin in wild-type and mutant strains of the alga, Cyanidium caldarium. Biochemistry. 1972 Nov 7;11(23):4235–4242. doi: 10.1021/bi00773a007. [DOI] [PubMed] [Google Scholar]


