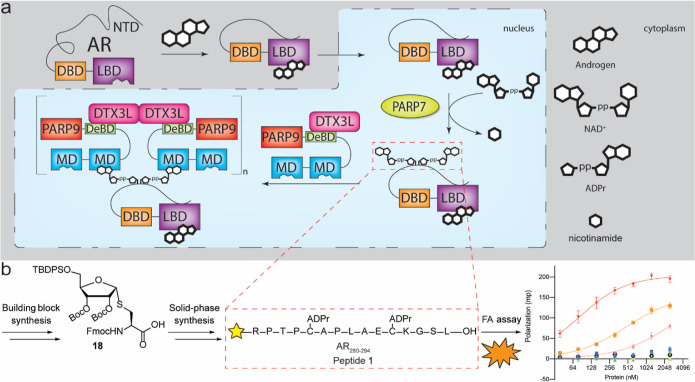
Figure 1.
(a) Proposed mechanism of AR-DTX3L/PARP9 binding. Binding of androgen to the LBD of AR facilitates a conformational change, enabling the unstructured NTD to interact with the LBD. Hereafter, the complex is transported to the nucleus, where it gets ADP-ribosylated by PARP7. After ADP-ribosylation, the agonist-bound AR is recognized by the MDs on PARP9 which exists as an oligomeric heterodimer with DTX3L, leading to the modulation of AR-dependent gene expression. (b) Workflow for obtaining binding affinity of model peptide 1. Solid-phase synthesis of dual-ADPr containing peptide 1 was based on building block 18. Solid-phase peptide synthesis gave peptide 1 which could be tested for its affinity toward the oligomeric, heterodimerized reader complex DTX3L/PARP9.