Abstract
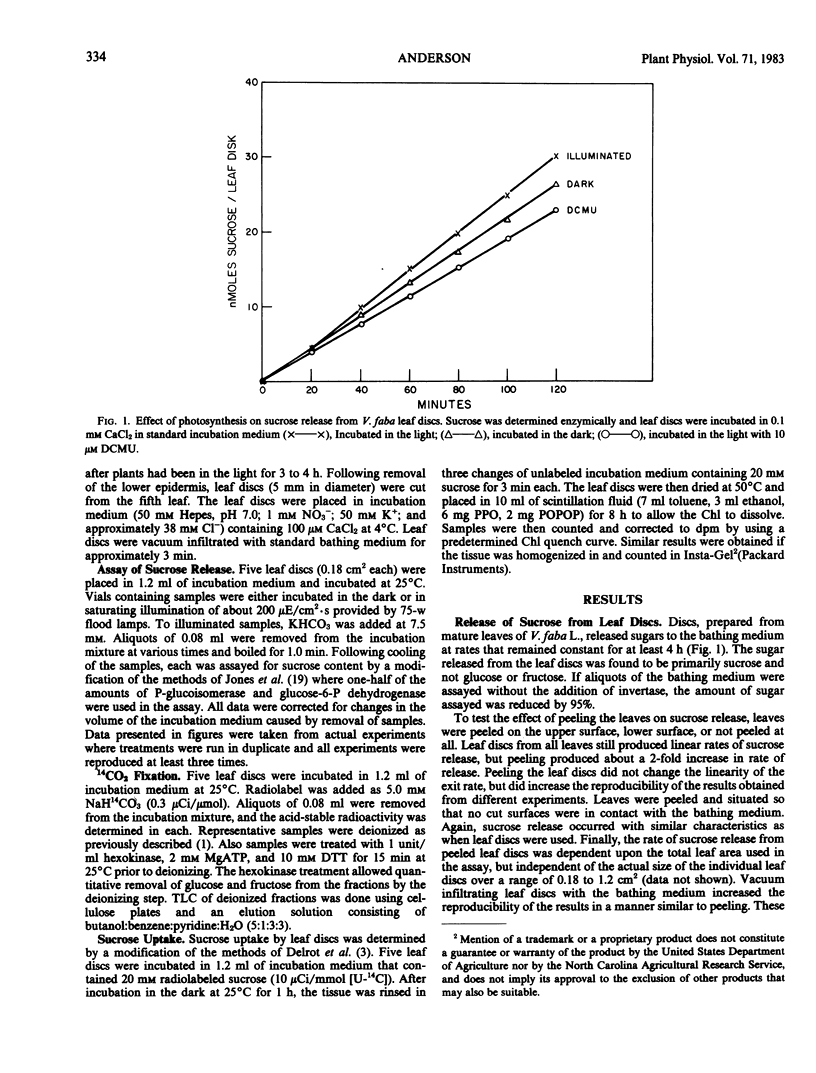
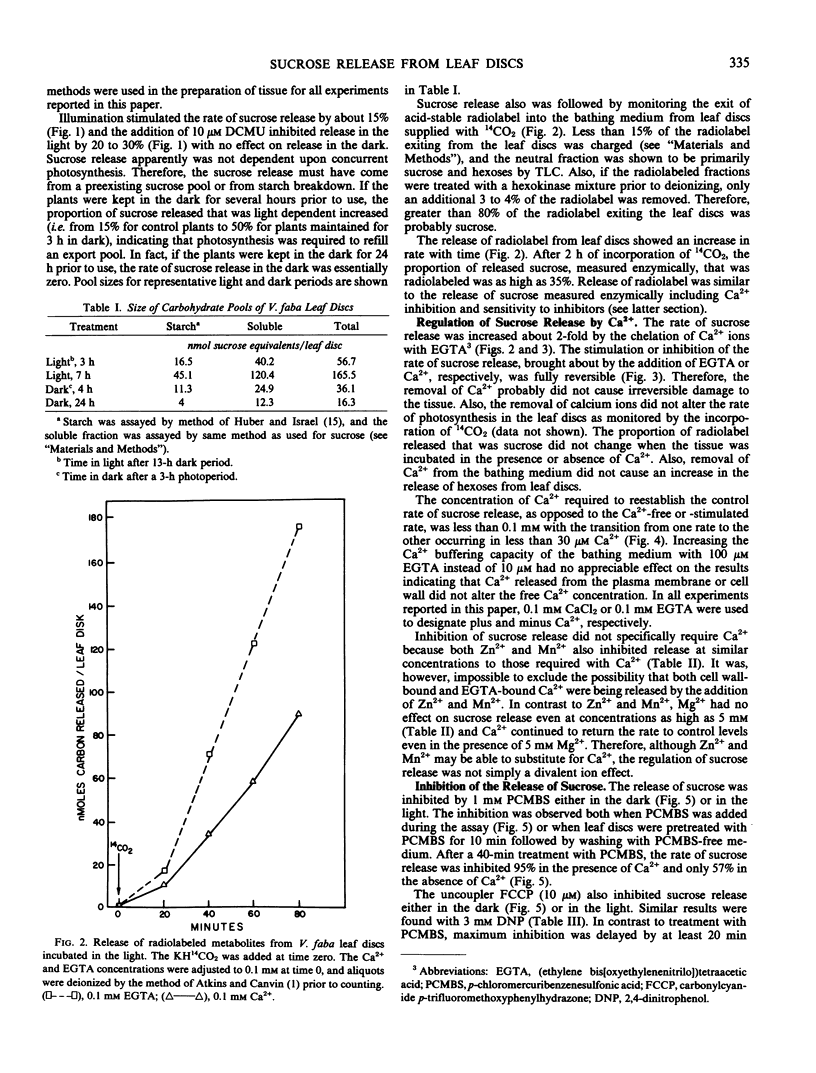
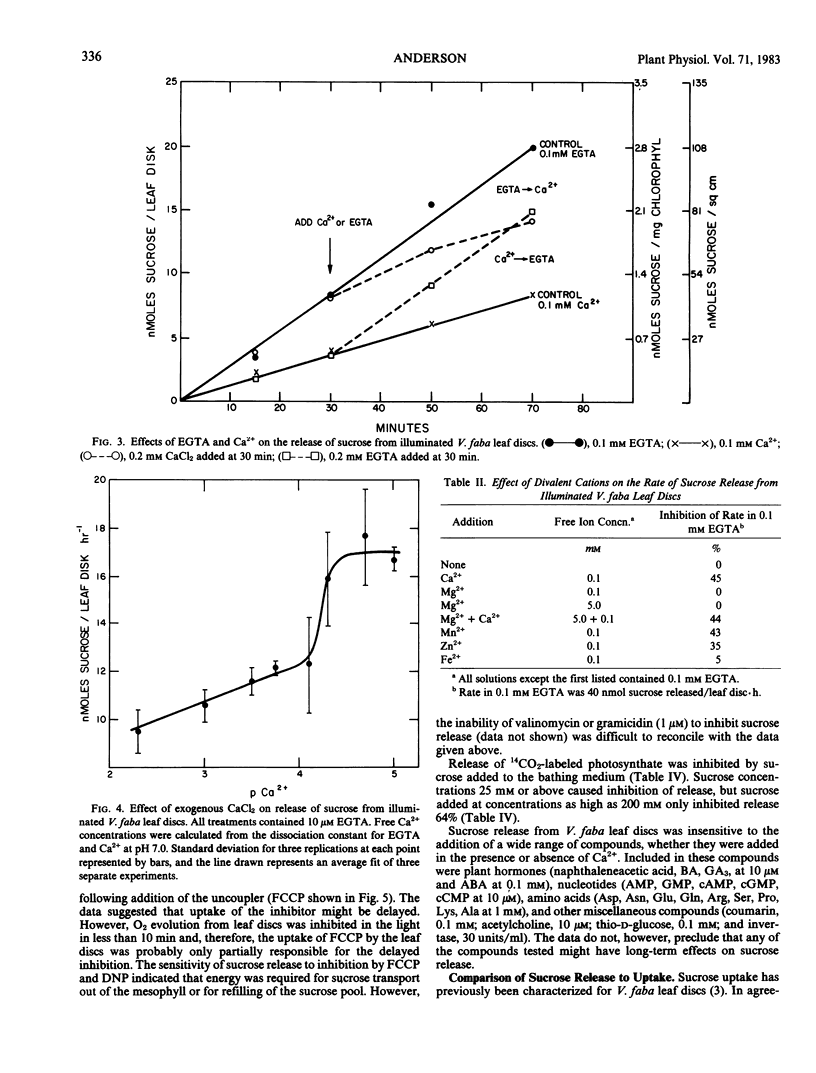
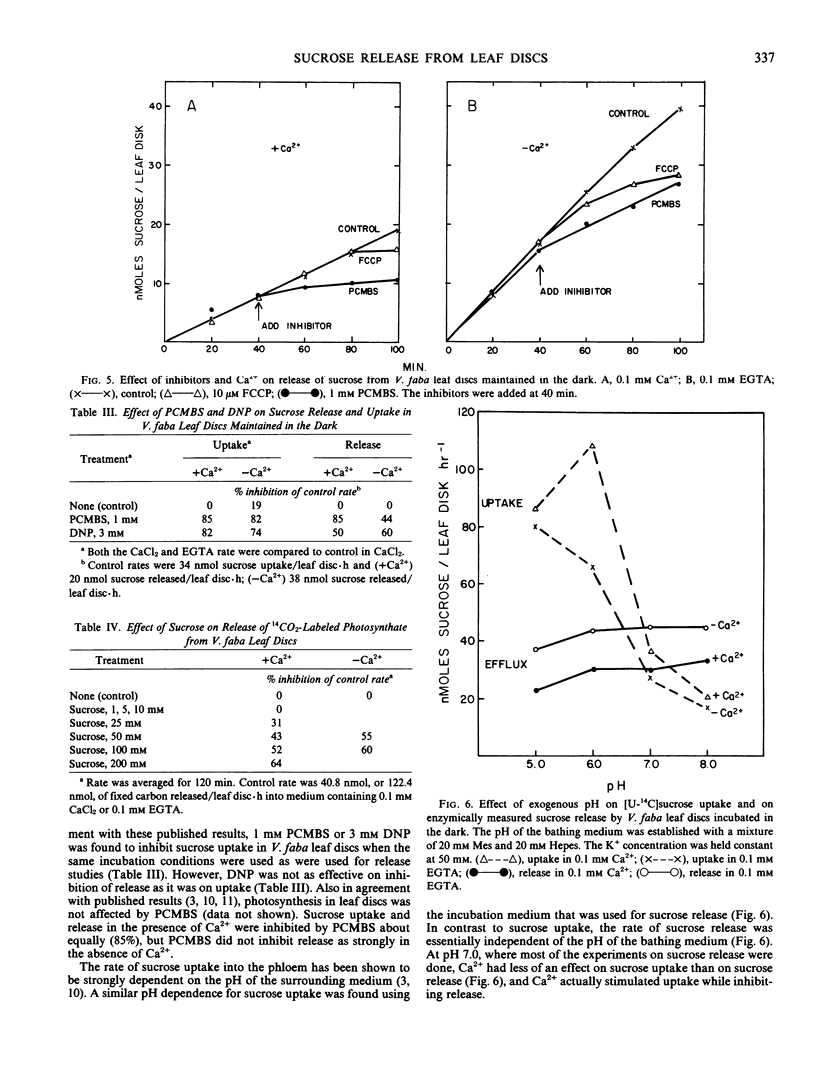
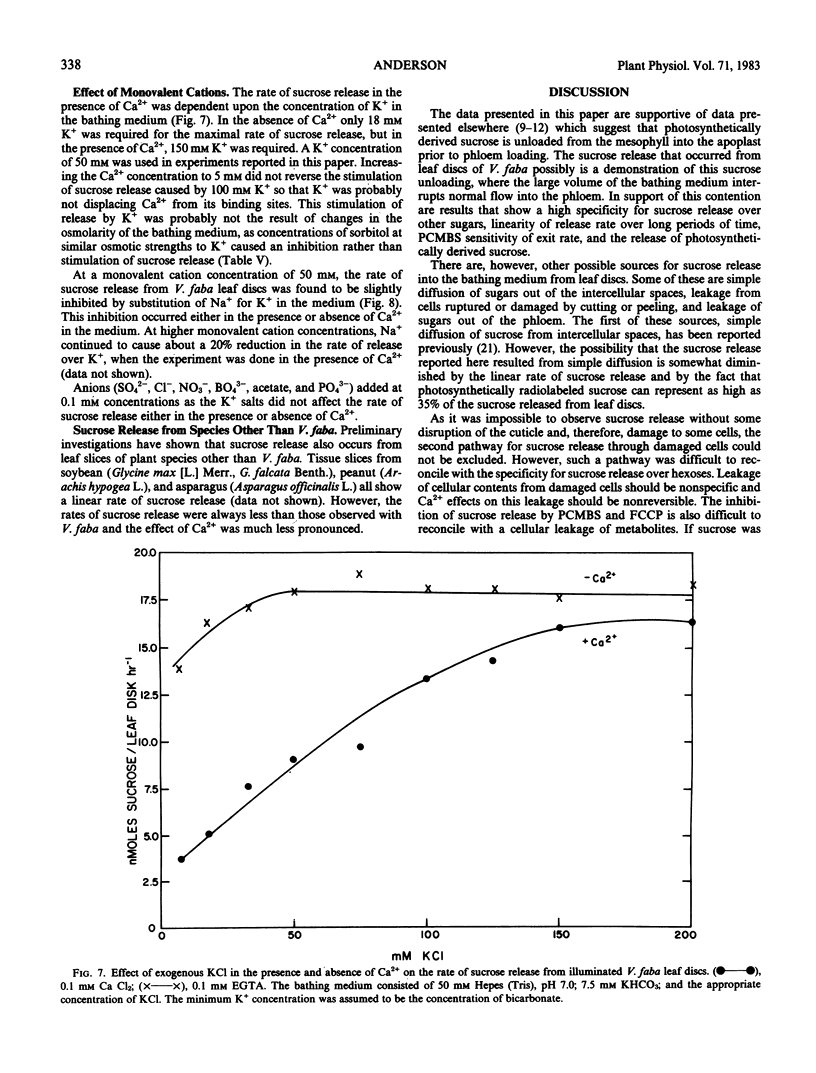
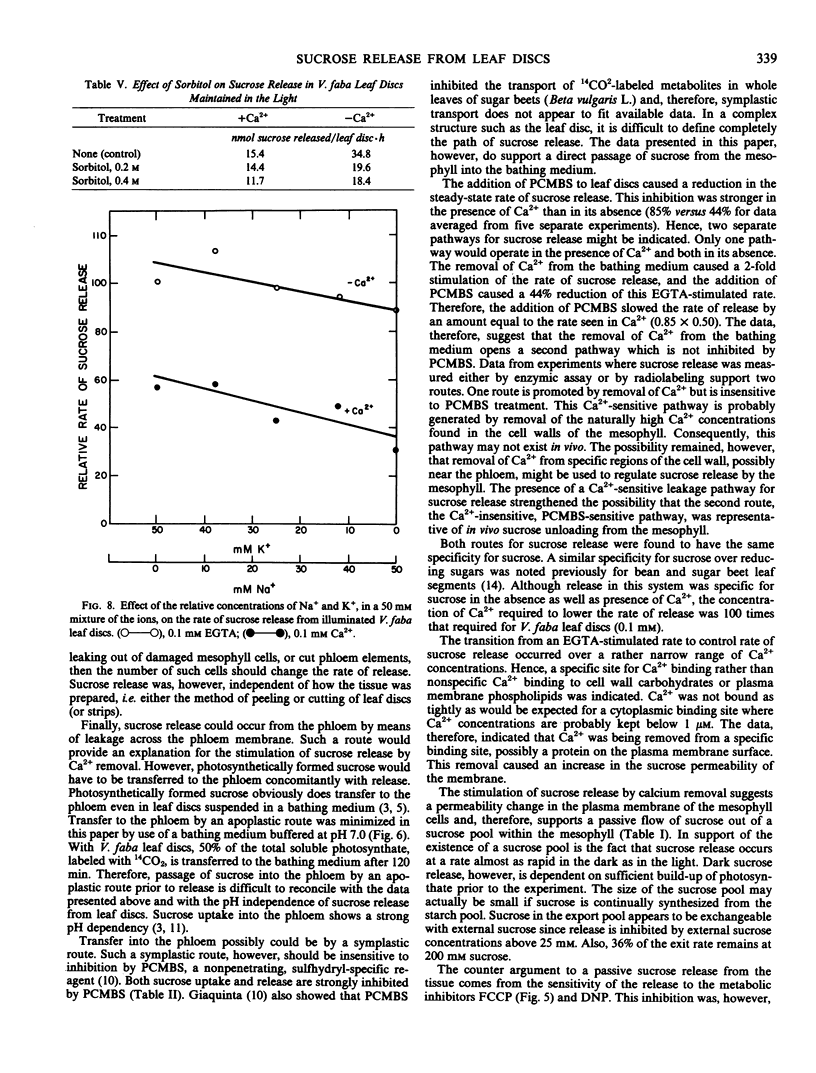
The release of sucrose from leaf discs of Vicia faba L. to a bathing medium was studied for evidence of a relationship between this release and mesophyll export of photosynthate in vivo. Sucrose was released specifically over hexoses and represented over 85% of total photosynthate released. The sucrose appeared to be derived from the mesophyll tissue directly and release did not require concurrent photosynthesis. The data indicated two separate channels for sucrose release. The first was sensitive to inhibition by 1 millimolar p-chloromercuribenzenesulfonic acid and the second was promoted by lowering the Ca2+ concentration below 0.1 millimolar. Flow through both channels was about equal when tissue that had been actively photosynthesizing for several hours was used. The rate of release was not dependent on the extracellular pH, but was inhibited by 10 micromolar carbonylcyanide p-trifluromethoxyphenylhydrazone. Lowering the Ca2+ concentration below 0.1 millimolar or raising the K+ concentration above 100 millimolar stimulated sucrose release. The stimulation by high K+ was not reversed by adding Ca2+. The data supported the postulate that Ca2+ removal or K+ addition changed the permeability of the mesophyll plasma membrane to sucrose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doman D. C., Geiger D. R. Effect of Exogenously Supplied Foliar Potassium on Phloem Loading in Beta vulgaris L. Plant Physiol. 1979 Oct;64(4):528–533. doi: 10.1104/pp.64.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Sugar Selectivity and Other Characteristics of Phloem Loading in Beta vulgaris L. Plant Physiol. 1977 May;59(5):953–960. doi: 10.1104/pp.59.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979 Apr;63(4):744–748. doi: 10.1104/pp.63.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley R., Metwally A., Overstreet R. Effects of Ca Upon Metabolic and Nonmetabolic Uptake of Na and Rb by Root Segments of Zea mays. Plant Physiol. 1965 May;40(3):513–520. doi: 10.1104/pp.40.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Moreland D. E. Co-transport of Potassium and Sugars across the Plasmalemma of Mesophyll Protoplasts. Plant Physiol. 1981 Jan;67(1):163–169. doi: 10.1104/pp.67.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Moreland D. E. Translocation: EFFLUX OF SUGARS ACROSS THE PLASMALEMMA OF MESOPHYLL PROTOPLASTS. Plant Physiol. 1980 Mar;65(3):560–562. doi: 10.1104/pp.65.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Moore D. P., Hannapel R. J. Role of Calcium in Absorption of Monovalent Cations. Plant Physiol. 1960 May;35(3):352–358. doi: 10.1104/pp.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S., Hanson J. B. The Effect of Calcium on Potassium Accumulation in Corn and Soybean Roots. Plant Physiol. 1957 Jul;32(4):312–316. doi: 10.1104/pp.32.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman R. H., Willis C. Correlation between the Suppression of Glucose and Phosphate Uptake and the Release of Protein from Viable Carrot Root Cells Treated with Monovalent Cations. Plant Physiol. 1971 Sep;48(3):287–293. doi: 10.1104/pp.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic salts on tissue permeability. Plant Physiol. 1976 Aug;58(2):182–185. doi: 10.1104/pp.58.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Jensen R. G. Metabolism of Separated Leaf Cells: III. Effects of Calcium and Ammonium on Product Distribution During Photosynthesis with Cotton Cells. Plant Physiol. 1973 Jul;52(1):17–22. doi: 10.1104/pp.52.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


