Figure 2.
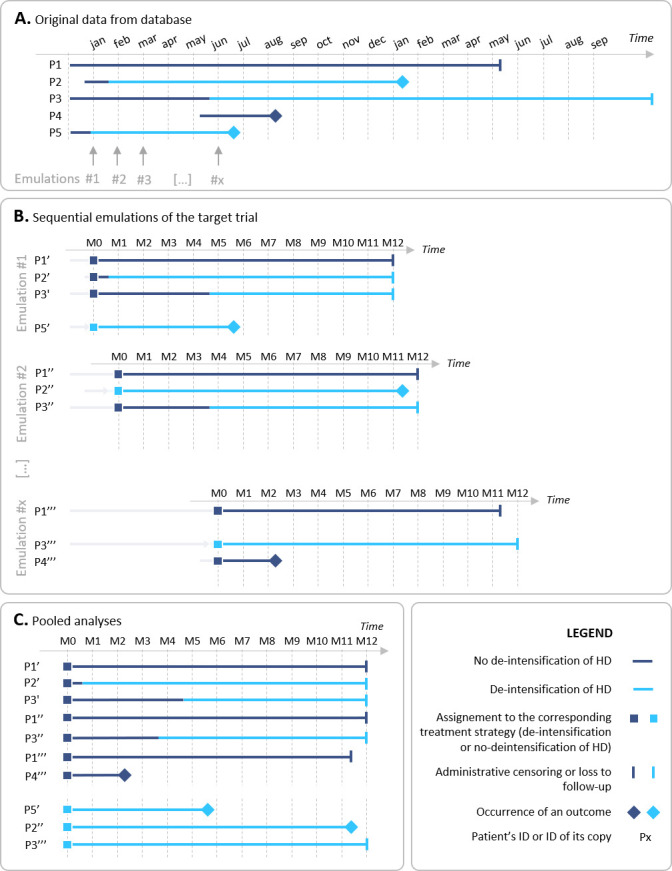
Theoretical example of the sequential emulations of the target trial. (A) In this theoretical example, five patients (P1–P5) were included from the database. Available data for these patients are represented on the timeline. (B) The target trial is sequentially emulated each month, and examples of these sequential emulations are provided for January (emulation #1), February (emulation #2), March (emulation #3), and June (emulation #x). For emulation #1, one cloned copy of patient (P5) started deintensification, which can be compared with three cloned copies of patients (P1, P2 and P3). If the cloned copies meet the eligible criteria of the target trial, they are kept for analysis. Treatment assignment is based on the prescription data compatible with the treatment strategy, and confounders at baseline of the sequential trial (ie, calendar month in the database) are also measured for each cloned copy (eg, comedication). To ensure coinciding of eligibility, treatment assignment and start of follow-up, thus avoiding immortal-time and selection biases,22 the follow-up of each cloned copy is restarted at the beginning of the sequential trial. For emulation #2, one cloned copy of patient P2 that started deintensification can be compared with two cloned copies of patients P1 and P3 that did not. Cloned copies of patients (P5″) are no longer eligible for the sequential emulated trial, according to eligibility criteria of the target trial: the patient already began deintensification in the past year (or in other words, a new-user design is applied). Assignment to treatment strategy, confounders measurement and follow-up reinitialisation are the same as for emulation #1. For emulation #3, no participant started deintensification in the database, so the comparison between deintensification and no deintensification is not feasible, and the cloned copies are not kept for analysis. These steps are repeated as many times as the target trial can be emulated, that is, each month when at least one eligible cloned copy starts the deintensification strategy and can be compared with a cloned copy belonging to the no-intensification arm. (C) Finally, the cloned copies of all sequential emulated trials are stacked in the final dataset for pooled analysis. HD, hypoglycaemic drug; M, months.
