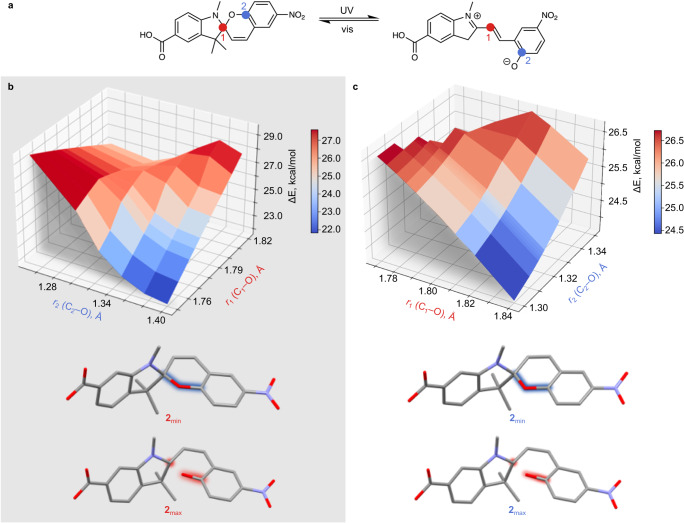
Fig. 6. Optimized structures of the spiropyran and merocyanine photoisomers of 2.
a The C1 and C2 atoms used for bond length monitoring are depicted as red (C1) and blue (C2) dots. b 3D PES for non-integrated 2 as a function of r1 and r2. c 3D PES for coordinatively-integrated 2 as a function of r1 and r2. The optimized geometrical conformations at the maximum and minimum energies for non-integrated and coordinatively-integrated 2 are highlighted in red and blue, respectively. Hydrogen atoms have been omitted for clarity. The maximum energy conformation for non-integrated 2 (b) occurs at r1 = 1.787 Å, r2 = 1.318 Å, and corresponding ∆E = 26.66 kcal/mol. The maximum energy conformation for coordinatively-integrated 2 (c) occurs at r1 = 1.781 Å, r2 = 1.318 Å, and ∆E = 26.57 kcal/mol.