Abstract
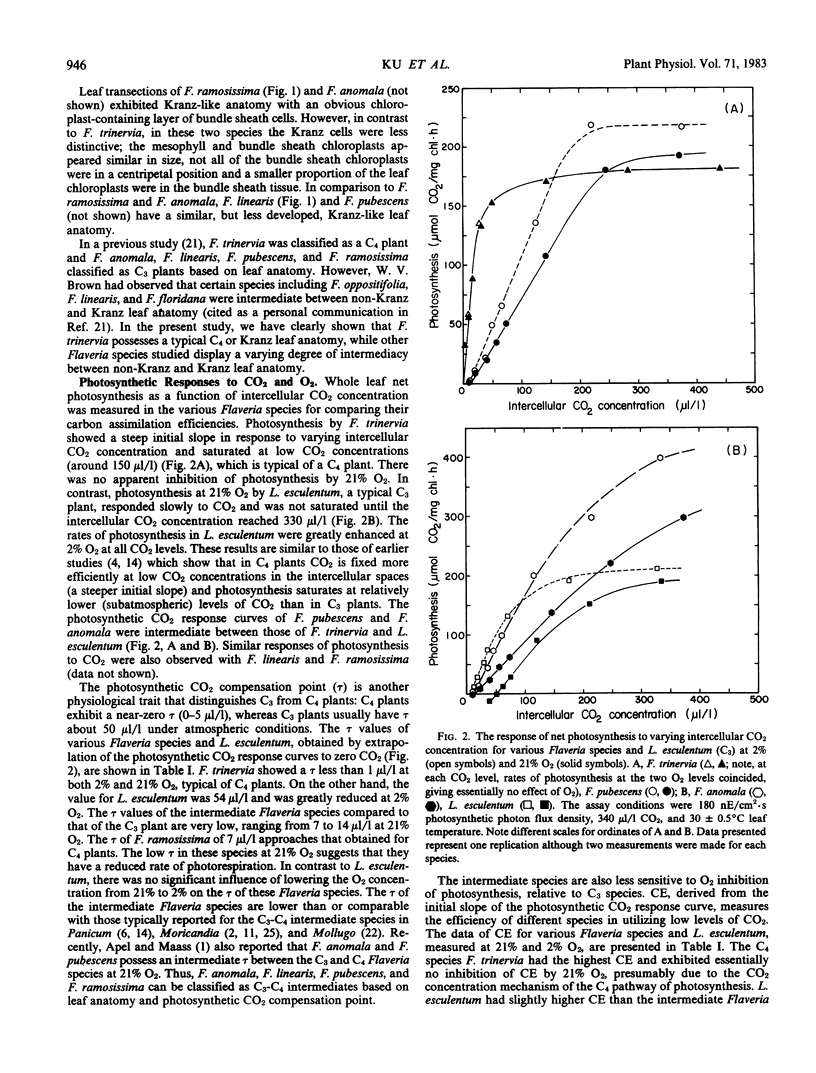
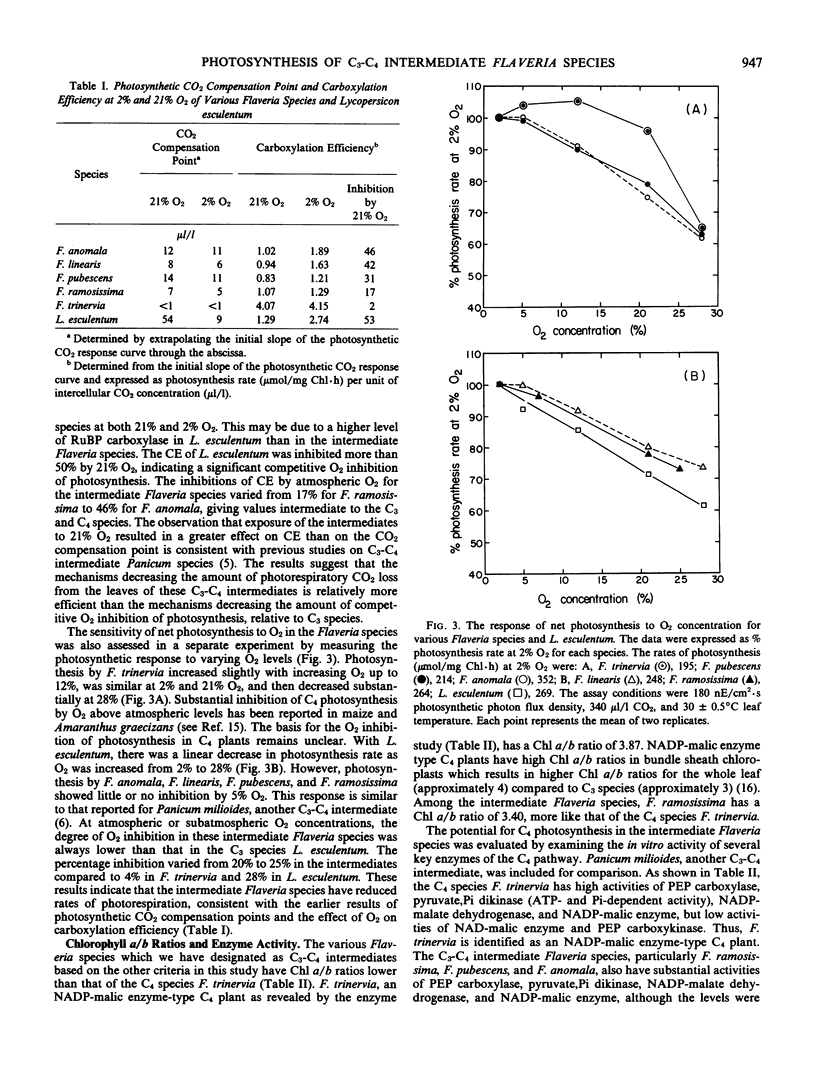
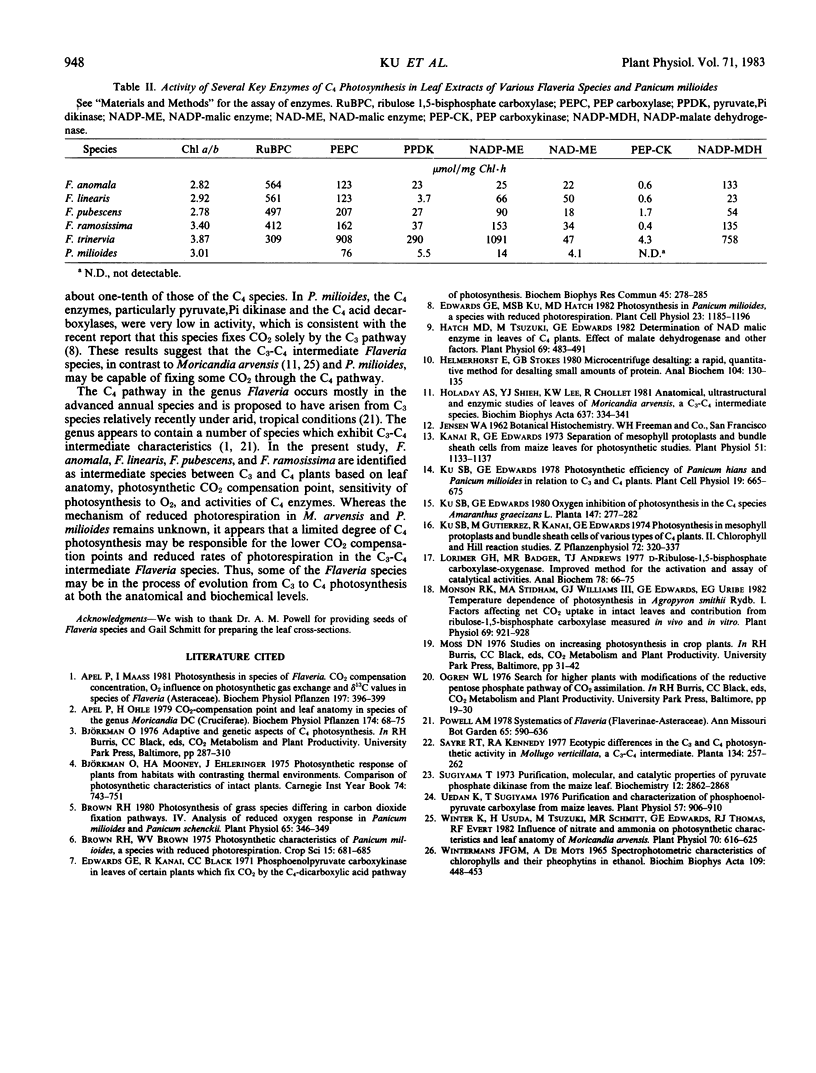
Four species of the genus Flaveria, namely F. anomala, F. linearis, F. pubescens, and F. ramosissima, were identified as intermediate C3-C4 plants based on leaf anatomy, photosynthetic CO2 compensation point, O2 inhibition of photosynthesis, and activities of C4 enzymes. F. anomala and F. ramosissima exhibit a distinct Kranz-like leaf anatomy, similar to that of the C4 species F. trinervia, while the other C3-C4 intermediate Flaveria species possess a less differentiated Kranz-like leaf anatomy. Photosynthetic CO2 compensation points of these intermediates at 30°C were very low relative to those of C3 plants, ranging from 7 to 14 microliters per liter. In contrast to C3 plants, net photosynthesis by the intermediates was not sensitive to O2 concentrations below 5% and decreased relatively slowly with increasing O2 concentration. Under similar conditions, the percentage inhibition of photosynthesis by 21% O2 varied from 20% to 25% in the intermediates compared with 28% in Lycopersicon esculentum, a typical C3 species. The inhibition of carboxylation efficiency by 21% O2 varied from 17% for F. ramosissima to 46% for F. anomala and were intermediate between the C4 (2% for F. trinervia) and C3 (53% for L. esculentum) values. The intermediate Flaveria species, especially F. ramosissima, have substantial activities of the C4 enzymes, phosphoenolpyruvate carboxylase, pyruvate, orthophosphate dikinase, NADP-malic enzyme, and NADP-malate dehydrogenase, indicating potential for C4 photosynthesis. It appears that these Flaveria species may be true biochemical C3-C4 intermediates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways: IV. ANALYSIS OF REDUCED OXYGEN RESPONSE IN PANICUM MILIOIDES AND PANICUM SCHENCKII. Plant Physiol. 1980 Feb;65(2):346–349. doi: 10.1104/pp.65.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Kanai R., Black C. C. Phosphoenolpyruvate carboxykinase in leaves of certain plants whick fix CO 2 by the C 4 -dicarboxylic acid cycle of photosynthesis. Biochem Biophys Res Commun. 1971 Oct 15;45(2):278–285. doi: 10.1016/0006-291x(71)90814-x. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Tsuzuki M., Edwards G. E. Determination of NAD Malic Enzyme in Leaves of C(4) Plants : EFFECTS OF MALATE DEHYDROGENASE AND OTHER FACTORS. Plant Physiol. 1982 Feb;69(2):483–491. doi: 10.1104/pp.69.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson R. K., Stidham M. A., Williams G. J., Edwards G. E., Uribe E. G. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : I. FACTORS AFFECTING NET CO(2) UPTAKE IN INTACT LEAVES AND CONTRIBUTION FROM RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE MEASURED IN VIVO AND IN VITRO. Plant Physiol. 1982 Apr;69(4):921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Usuda H., Tsuzuki M., Schmitt M., Edwards G. E., Thomas R. J., Evert R. F. Influence of Nitrate and Ammonia on Photosynthetic Characteristics and Leaf Anatomy of Moricandia arvensis. Plant Physiol. 1982 Aug;70(2):616–625. doi: 10.1104/pp.70.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]



