Abstract
Diabetes is an endocrine illness involving numerous physiological systems. To understand the intricated pathophysiology and disease progression in diabetes, small animals are still the most relevant model systems, despite the availability and progression in numerous invitro and insilico research methods in recent years. In general, experimental diabetes is instigated mainly due to the effectiveness of animal models in illuminating disease etiology. Most diabetes trials are conducted on rodents, while some research is conducted on larger animals. This review will discuss the methodology and mechanisms in detail for preparing diabetic animal models, considering the following important points. The exact pathophysiology of the disease may or may not be replicated in animal models, the correct induction doses must be given and the combination of different approaches for the models is recommended to get desired results.
-
•
Animal models are essential to understand diabetes etiology and pathophysiology.
-
•
Diabetic models can be developed in both rodents and non-rodents.
-
•
Chemically induced and genetic models of diabetes are widely used to study diabetes and diabetic complications.
Keywords: Diabetic animal models, Diabetic ulcers, Rodents, Chemically induced, Genetic model
Method name: Development of diabetic animal models
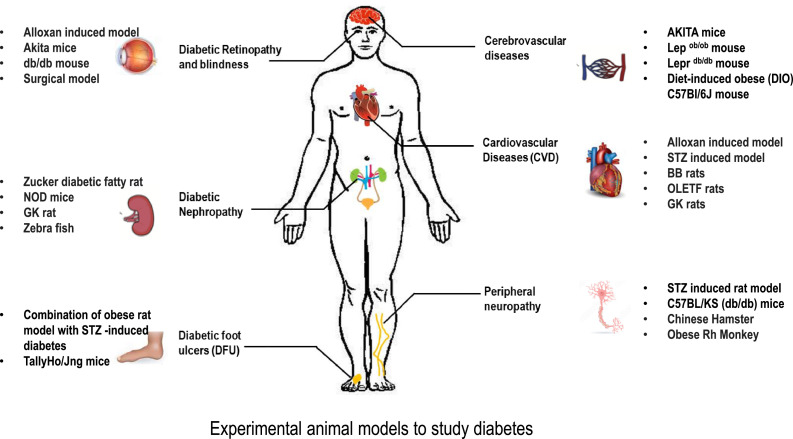
Graphical abstract
Experimental models to study diabetes and diabetic complications.
Specifications table
| Subject area: | Pharmacology, Toxicology and Pharmaceutical Science |
| More specific subject area: | Diabetes |
| Name of the reviewed methodology: | Development of diabetic animal models |
| Keywords: | diabetic animal models, diabetic ulcers, rodents, chemically induced, genetic model |
| Resource availability: | The data can be found from the literature available in Pubmed /Springer /Elsevier /Nature /Scopus |
| Review question: | Animal models for diabetes |
Method details
Introduction
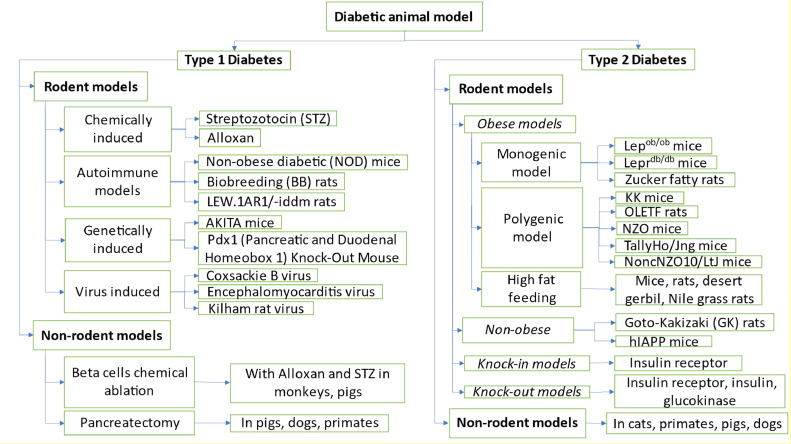
Diabetes, one of the most widespread and complex metabolic disorders, is a challenging global public health issue characterized by hyperglycemia, a condition with a high blood plasma glucose level. Chronic disease develops either when sufficient insulin (a hormone that controls blood sugar) is not produced by the pancreas or the insulin produced is not utilized by the body [1]. Based on the etiology and clinical characteristics, diabetes is typically divided into type 1, 2, and gestational diabetes. Genetic predisposition with several environmental factors is supposed to be the primary etiology of type 1 diabetes (T1D). Contrarily, insulin resistance is the primary contributor to type 2 diabetes (T2D), also known as adult-onset diabetes or non-insulin-dependent diabetes [2], [3], [4]. Diabetes poses a significant threat to one's health and burdens society financially. Long-term complications of diabetes include diabetic foot ulcers (DFU), diabetic neuropathy, diabetic retinopathy, diabetic nephropathy, and diabetic cardiomyopathy. Management and treatment of diabetes and diabetic complications are among the core research areas in biomedical sciences. To create novel and efficient methods of treating diseases like diabetes, research with animal models for preclinical studies is fundamental [5]. Animal models have been long used to understand diabetes and also to develop and test novel therapeutic products to treat diabetes and diabetic complications [6,7]. This review will highlight the methods for the development of different diabetic models. These models range from chemically induced diabetic models to genetic models with their associated advantages and disadvantages (Fig. 1).
Fig. 1.
Highlights the various experimental models used to study diabetes and diabetic complications.
Animal models
Diabetes is a major reason that causes heart attacks, blindness, kidney failure, stroke, and lower limb amputations. Animal models are the most helpful platform for understanding the complicated etiology, pathophysiology, and complex interactions in diabetes, despite the availability of newer methods in recent years. In animals, experimental diabetes mellitus is instigated, mainly due to its effectiveness in illuminating disease etiology and pathology. Diabetes related experiments are conducted on rodents as well as on larger animals.
Regarding diabetes, rodents are frequently utilzed as an appropriate animal model to understand the metabolic changes and pathophysiology involved in various stages of diabetes [8]. The rodents offer several favorable conditions and benefits over other species as an animal model of human disease and its treatments [9]. Experimental research using rodents is crucial to better understand the mechanism of insulin resistance and the potential treatments for diabetes and diabetic complications. The findings from these studies are faithfully applied to people with the disease [10,11]. Other than that, non-rodents such as pigs, rabbits, rhesus monkeys, and zebrafish have been utilized to study diabetes and diabetic complications.
The type of diabetes that the experimental animal models replicate, as well as the mechanism of induction, such as spontaneous (naturally occurring), induced (by chemical/drug/ physical), or by genetic changes (transgenic models), are used to categorize them [12,13]. Here are a few frequently utilized models for creating diabetes-like animals that are more effective in discovering novel therapeutics.
T1D animal models
An autoimmune attack on the pancreatic beta cells, which prevents the pancreas from producing sufficient insulin, is the primary feature of type 1 diabetes. The deficiency of insulin production is induced in animal models using a variety of methods, including methods to produce animals that develop autoimmune diabetes on their own or by chemically killing the pancreatic beta cells.
Chemically induced diabetes
For creating chemically induced diabetes animal models, streptozotocin (STZ) and alloxan are the two common substances.
Streptozotocin induced models
A glucosamine nitrosourea molecule known as streptozotocin (STZ) is a member of the class of chemotherapeutic alkylating agents [14]. To induce diabetes in laboratory animals, the STZ is the most frequently utilized chemical. Mice and rats have been used frequently to induce diabetes using STZ because of their small size, simplicity of handling, and affordability.
Mechanism of action:
STZ is selectively taken up by pancreatic β-cells through the GLUT-2 (a transmembrane carrier protein) transporter causing its destruction.
-
•
At low doses (often administered as multiple exposures of dosages 20–40 mg/kg/day) as a result of the release of the glutamic acid decarboxylase enzyme, STZ causes an immunological and inflammatory response. In autoimmune diabetes, this enzyme is a key autoantigen [15,16].
-
•
In the high-portion STZ approach, a single dose of STZ (100–200 mg/kg for mice or 35–65 mg/kg for rats) is administered intravenously or intraperitoneally to the animals, resulting in significant pancreatic beta-cell death but little to no insulin production [17].
-
•
For developing diabetic complications such as ulcers and neuropathy, animals are kept in diabetic conditions for 2–8 weeks once diabetes is induced.
Advantages:
-
•
It is a dependable model for diabetes research.
-
•
It can induce diabetes in rats, mice, and other animals like rabbits, guinea pigs, and monkeys. But STZ's ability to cause diabetes varies depending on the dosage, animal's species, strain, gender, and age [18,19].
-
•
STZ-induced diabetes in cats is similar to type 2 diabetes while similar to type 1 diabetes in rats [20].
Limitations:
-
•
Due to the significant mortality rate of this model, more animals are needed.
-
•
The animals can revert to normal glucose levels after a few weeks.
Alloxan induced models
Alloxan (5,5-dihydroxyl pyrimi-dine-2,4,6-trione) is a cytotoxic glucose analog [21]. It has been widely accepted that it selectively destroys the insulin-producing pancreatic beta-cells, thus used in laboratory animals to induce diabetes.
Mechanism of action:
Alloxan's ability to cause diabetes is mainly related to its quick absorption by beta cells and the production of free radicals that break beta cell DNA [22]. Other causes of beta cell injury by alloxan include oxidation of important -SH groups, including those of glucokinase [23], and disruptions in intracellular calcium homeostasis [24]. Depending on the strain and the mode of administration, doses for mice vary from 50 to 200 mg/kg and for rats from 40 to 200 mg/kg [25]. Long-term diabetes models in rabbits are usually administered a dosage of 100 mg/kg [26].
Advantages:
-
•
Quick and reasonably priced way to simulate diabetes.
-
•
Easily reproducible method of inducing diabetes in experimental animals.
Limitations:
Alloxan has a relatively narrow diabetogenic dose, and other body organs may be damaged when exposed to alloxan [25].
The key details along with age and dosage requirements of the chemically induced diabetic models is represented below (Table 1).
Table 1.
Table representing dosage requirement for a chemical-induced diabetic model in different species.
| Chemical | Animal model | Age of animals | Dose required | Frequency of dose required | Refs. |
|---|---|---|---|---|---|
| Streptozotocin | Rat | 8–12 weeks | 35–65 mg/kg i.v./i.p. | single dose is common. | [14] |
| Mice | 8–12 weeks | 100–200 mg/kg i.v./i.p. | single | ||
| Pigs, cynomolgus monkey | Can vary, generally young or adolescent. | 150 mg/kg i.v. or multiple doses | single/multiple depending on research objective. | [15] | |
| Alloxan | Rat | 8–12 weeks | 40–200 mg/kg i.v./i.p. | single | [22] |
| Mice | 8–12 weeks | 50–200 mg/kg i.v./i.p. | single | ||
| Rabbit | Can vary, usually are young or young adults. | 100 mg/kg i.v. | single/multiple, depends on research goals. | [23] | |
| Yucatan mini pig | Can vary, generally young or adolescent. | 175 mg/kg i.v. | single/multiple depending on experimental design. | [43] |
Autoimmune models
The Non-Obese Diabetic (NOD) Mouse and the Biobreeding (BB) Rat are the most often utilized autoimmune model of type 1 diabetes [27]. Another model, LEW.1AR1/-iddm rats, is also identified for spontaneous autoimmune models of type 1 diabetes [28].
NOD mice:
Makino and colleagues in 1980 created the NOD strain at Shionogi Research Laboratories in Aburahi, Japan. The cataract-prone strain of JcI: ICR mice were outbred by the group to create the NOD strain [29].
Mechanism of action:
In the NOD mouse, diabetes development is characterized by insulitis, a condition where leukocytes infiltrate the pancreatic islets [30]. Diabetes is more common in females colonies than males. Even though diabetes can develop up to 30 weeks, most pancreatic insulin is usually lost when the insulitis triggers beta cell loss at around 10 to 14 weeks [31].
Advantages: These models enable control over the autoimmune response's timing.
Limitations:
-
•
Disease onset is unpredictable due to gender differences.
-
•
Compared to chemically induced diabetes, these models are more expensive to maintain as a model of T1D.
-
•
Translation of tested therapy is difficult [32].
-
•
Requirement of specific pathogen-free conditions.
BB rat:
From outbred Wistar rats, BB rats were developed. First discovered in a Canadian colony in 1974, spontaneous autoimmune diabetes resulted in the development of two founder colonies, one inbred biobreeding diabetes-prone/worceste (BBDP/Wor) and another outbred biobreeding diabetes-prone (BBdp), from which all substrains have descended. Additionally, to serve as controls, diabetes-resistant BB rats have been created [32].
Mechanism of action:
Diabetes in BB rats often appears shortly after puberty and occurs equally frequently in males and females. Rats between the ages of 8 and 16 weeks develop diabetes in about 90 % of cases [33].
Advantages: The model is beneficial for understanding T1D genetics [34].
Limitations: There are signs of lymphopenia, which are not usual for T1D in humans [32].
LEW-iddm rats:
In a colony of Lewis congenic rodents that spontaneously emerged, the LEW-iddm rodent model for T1D is characterized by MHC Lewis.1AR1 (LEW.1AR1) haplotype.
Mechanism of action:
These rats certainly develop diabetes between 60 and 90 days, as evidenced by their rapid insulitis progression and widespread cell apoptosis [28]. Male and female experimental rat models of diabetes get the disease equally frequently [35].
Advantages:
-
•
LEW-iddm rat does not exhibit other autoimmune diseases, unlike the NOD mouse and BB rat models.
-
•
Survives well beyond the appearance of overt diabetes, making it useful for the study of diabetic consequences [36].
Limitations: More expensive as compared to chemically induced models.
Genetically induced insulin-dependent diabetes
A spontaneous mutation in C57BL/6NSlc animal gave rise to the AKITA mouse in Akita, Japan, which has an insulin 2 gene which prevents pro-insulin from being processed correctly.
Mechanism of action:
Overproduction of improperly folded proteins and ER stress leads to diabetes. As a result, three to four weeks old develop severe insulin-dependent diabetes characterized by polyuria, polydipsia, hypoinsulinemia, and hyperglycemia. For disease manifestation, direct infection of the pancreatic beta cells or an autoimmune reaction against the beta cell can result in destruction [37].
Advantages:
-
•
It can be utilized as a model for type 1 macrovascular diabetes [38].
-
•
It can be utilized as a model for diabetic neuropathy.
Limitations: Rarely survive past 12 weeks with disease untreated.
Virus-induced models of diabetes
In numerous animal models, viruses have been utilized to start beta cell death. The contribution of viruses to the etiology of type 1 diabetes is uncertain. Some of them are the coxsackie B virus, the encephalomyocarditis (EMC) virus, and the Kilham rat virus.
Mechanism of action:
The enterovirus coxsackievirus B4 (CVB4) is the most frequently found in diabetics. This CVB strain was found in the pancreas of a child diagnosed with diabetic ketoacidosis. On isolation, when injected into murine cells, it causes diabetes [39]. In mice, the EMC virus can infect and kill the pancreatic beta cells, leading to insulin-dependent hyperglycemia [40]. Kilham's rat virus (KRV), isolated from a naturally diabetic rat, reproducibly caused diabetes in naive diabetes-resistant (DR) BB/Wor rats. The disease's process appears to be indirect, involving stimulating immune cells rather than the virus directly infecting the beta cells [41].
Limitations:
-
•
Because the disease outcome depends on the virus's replication power and the infection's timing, the virus-induced model's development can be challenging.
-
•
Specific breeding facilities with higher biosafety levels.
Non-rodent animal models
Along with the well-researched and routinely used rodent models of type 1 diabetes, animal models from higher species have also been created. Developing spontaneous diabetes in these large animal models is highly uncommon and unpredictable. The two most prevalent ways to cause insulin dependency in such models are pancreatectomy and chemical treatment of pancreatic beta cells with STZ and alloxan.
Pancreatectomy:
Inducing hyperglycemia in pigs, dogs, and primates through performing pancreatectomy has been done by many researchers to study diabetes [42], [43], [44].
Limitations:
-
•
It is very invasive.
-
•
Chances of hypoglycemia are higher.
-
•
Pancreatic exocrine function is profoundly affected in animals.
Pancreatic beta cells chemical ablation:
The beta cell toxicity of STZ and alloxan varies between species. The rat can develop diabetes irreversibly with an STZ dose of 50 mg/kg, whereas cynomolgus monkeys and pigs need a larger dose (150 mg/kg) [18]. In some higher animal models, researchers also combine partial pancreatectomy with STZ therapy, thus lowering the dose of STZ [44,45]. Alloxan (175 mg/kg intravenously) was administered to develop diabetes in a Yucatan mini pig model at the University of Missouri at Columbia [46].
An invertebrate animal model can be substituted to get around the issues with contemporary animal rights and lower the mortality rate of mammals. Attempts have been made using silkworm as a diabetic model. The signaling system that controls blood glucose levels in humans is similar to the signaling mechanism that controls hemolymph levels in silkworms. This can be accomplished by expressing the human insulin receptor (hIR) in transgenic silkworms [47]. Zebra fish are now frequently used in diabetes research due to fully sequenced genome availability, ease of genetic manipulation, and excellent fertility rates, serving as a unique model for studying human metabolic problems [48].
T2D animal models
Most type 2 diabetes animal models used today are obese because type 2 diabetes and obesity are strongly related. Genetic engineering or naturally occurring mutations may result in obesity. As an alternative, obesity can be induced with a high-fat feeding diet for experimental animals. This section will explain the model method and conditions where it can be used to study T2D.
Monogenic models of obesity
The leptin signaling is faulty in the most popular monogenic models of obesity. Since leptin promotes satiety, an absence of functioning leptin in these animals results in hyperphagia and ensuing obesity. These models include the leptin-deficient Lep ob/ob mouse, the leptin receptor-deficient Zucker Diabetic Fatty rat, and the Lepr db/db mouse.
Advantages: To evaluate novel treatments for type 2 diabetes, these models are frequently employed due to their pathophysiology matching human patients.
Lep ob/ob mouse
These were discovered at Jackson Laboratory in 1949 from a spontaneous mutation of leptin protein in an outbred colony of C57BL/6 mice.
Mechanism of action:
The mice gain weight at the age of 2 weeks and develop hyperinsulinemia. Blood glucose levels start to rise at four weeks and peak at three to five months, indicating hyperglycemia [49].
Limitations:
Lepr db/db mouse
A leptin receptor autosomal recessive mutation gave rise to the Lepr db/db mouse, which was developed at the Jackson Laboratory [52].
Mechanism of action:
The C57BLKS/J is the background that is most frequently utilized. Obesity is visible from 3 to 4 weeks of age, hyperinsulinemia at 2 to 4 weeks, and the onset of hyperglycemia at 4 to 8 weeks [53].
Limitations: Relative short lifespan.
Zucker Diabetic Fatty rat
Inbred Zucker Diabetic Fatty Rats were created due to a mutation in Zucker Fatty Rats, first identified in 1961 due to a cross between Sherman and Merck M-strain rats.
Mechanism of action:
The defective leptin receptor induces hyperphagia [54]. ZDF rats have higher levels of apoptosis in the beta cells, which prevents them from compensating for their more severe insulin resistance. This condition is characterised by hyperinsulinemia at roughly 8 weeks of age, followed by a drop in insulin levels. In male rats, diabetes often appears at 8 to 10 weeks; in contrast, females do not develop overt diabetes [53,55,56].
Limitations: Contrary to human heterogeneity, diabetes is characterized by a largely inbred, homogeneous, and predominantly monogenic inheritance pattern.
Polygenic models of obesity
Polygenic models form another valuable system for studying diabetes, glucose intolerance, and obesity. Investigations that sought to alleviate the signs of type 2 diabetes have used these polygenic animals in many studies.
Advantages:
-
•
Gives a more realistic representation of the human condition.
-
•
It can be used to study various diabetic complications, such as diabetic nephropathy.
-
•
Used for studying and evaluating treatment strategies for diabetic wound healing.
KK mice: Kondo developed KK mice from wild-derived ddY mice in Japan in 1957. They are a mildly obese and hyperleptinemic strain [57].
OLETF rats (Otsuka Long-Evans Tokushima Fat) were created using selective breeding from the spontaneously diabetic rat found in an outbred colony of Long Evans Rats in 1984 by the Tokushima Research Institute. This strain shows features with mild obesity and late-onset hyperglycemia (after 18 weeks). Diabetes is inherited by the males [58].
NZO (New Zealand Obese) mice is a polygenic model of obesity developed through selective breeding. Due to leptin resistance, it is hyperphagic and obese, and by 9–12 weeks of age, these mice are hyperleptinaemic and also hyperinsulinaemic [59].
TallyHo/Jng mice are a naturally occurring model of obesity and type 2 diabetes created through selective breeding of mice that naturally acquired hyperglycemia and hyperinsulinemia in an outbred colony of Theiler Original mice. The development of hyperglycemia, which only affects male mice, begins between 10 and 14 weeks [60,61].
NoncNZO10/LtJ mice were produced by crossing non-obese non-diabetic mice (NON/Lt) with independent quantitative trait loci (QTLs) that confer diabetes risk in two unrelated strains of NZO mice. These mice begin to exhibit insulin resistance in their skeletal muscles, liver, and liver at 8 weeks, and by 12 weeks, they are exhibiting chronic hyperglycemia [61,62].
Models based on high-fat diet feeding
In 1988, the first description of the high-fat feeding model for C57BL/6 mice appeared. High-fat intake can result in obesity, hyperinsulinemia, and impaired glucose homeostasis. In place of standard chow, a diet with a significantly higher percentage of calories from fat is fed to the animals. The amount consumed should be monitored regularly to prevent compensation of diet by eating less food. Within a week of beginning the high-fat diet, mice fed with high fats can weigh more than mice fed chow. Weight gain is linked to insulin resistance, and a lack of compensatory beta cells results in reduced glucose tolerance. Compared to genetic models of obesity-induced diabetes, environmental factors such as obesity are regarded to model the human condition more closely. In transgenic or knock-out animals, high-fat feeding is frequently used. Under normal circumstances, these models may not express an obvious diabetes phenotype, but when the beta cells are pushed, it may become clear that the overlap with the human condition is significant [63,64]. High-fat diet with the chemical destruction of beta cells using STZ or alloxan is also being utilised to study diabetes and its complications.
New-generation models
CRISPR_Cas9:
The lengthy backcrossing processes used in conventional procedures to create genetically altered NOD mouse models took many years and frequently led to the survival of strain-specific alleles close to the intended mutation site. But the development of CRISPR/Cas9 technology, as Ratiu and colleagues showed, has completely changed this procedure. Researchers can shorten the time it takes to generate a model from years to just a few months by directly modifying the NOD genome and avoiding the requirement for significant backcrossing. As seen by their work on B-cell pathways, this ground-breaking method provides a quicker and more accurate means to research diseases [65].
Microbiome-modified models:
The NOD mouse and BB rat are two diabetic animal models that have emphasised the critical function of the gut microbiota in autoimmune and metabolic illnesses. These models demonstrate how the gut microbiota has a significant impact on energy metabolism and obesity, with germ-free animals being protected from diet-induced weight gain. Through AMP-activated protein kinase (AMPK) and peroxisomal proliferator-activated receptor gamma coactivator-1alpha (Pgc-1alpha), the microbiome influences important metabolic pathways, including fatty acid metabolism. Furthermore, it is crucial for the emergence of type 1 diabetes, with specific bacterial strains, including Lactobacillus species, influencing the risk of the condition. These findings highlight the potential for microbiome-based therapies in diabetes research, presenting promising lines of defence against and avenues for curing the disease [66,67].
Humanized mouse models:
To study the pathophysiology of diabetes and test prospective treatments, immunodeficient mice are grafted with viable human cells and tissues to create humanised diabetic animal models. The shortcomings of conventional rodent models, which are unable to accurately predict the results of human clinical trials, are addressed by these models. These models are developed using specialised strains of immunodeficient mice, such as NOD-scid IL2rγnull (NSG) mice. These mice support a high engraftment of human cells and organs, including human islets, hematopoietic stem cells, and immune systems, while having nonfunctional immune systems.
Specialized mouse strains, such as NOD-Rag1null IL2rγnull Ins2Akita (NRG-Akita) mice with the Ins2Akita mutation, are used in diabetes research to imitate spontaneous hyperglycemia, making them appropriate for evaluating human islet transplants and researching immunological responses. Other models, such as NSG Tg (RIP-HuDTR) mice, provide more controlled research environments by targeting destroying mouse beta cells to cause hyperglycemia at will. These humanised mice models enable in vivo examination of human beta cells during autoimmune or alloimmune attacks, assisting in the exploration of the pathophysiology of diabetes, identifying prospective therapeutic targets, and assessing promising preclinical drugs prior to human clinical trials [68].
The following chart offers a concise overview of the models featured in the article (Chart 1).
Chart 1.
Summary of the frequently utilized diabetic animal models.
Challenges and way ahead
In order to better understand both normal and pathological processes across species, comparative medicine, and animal models will continue to be potent tools. They will also make it easier to move preclinical research into clinical settings, ultimately enhancing human health. However, a single animal model cannot accurately simulate all disease occurrences. Furthermore, animal models frequently fall short of accurately simulating the full variability and complexity of human diseases, particularly in complex situations like diabetes. This article suggests that choosing an appropriate animal model for diabetes, as opposed to one that has been used before or is often used, may be a better option. Laboratory animal genetic uniformity, varied immune reactions, and the final failure of promising medicines in human clinical trials highlight the need for careful interpretation and thorough confirmation of animal model results in the context of clinical applications.
This article has discussed the realistic and suitable diabetic models for the study of common complications of diabetes, such as neuropathy or nerve damage, ulcers, to heart disease or macrovascular abnormalities, nephropathy, or kidney disease. It also describes the methodologies for the construction of various diabetic models. Animal models for diabetes can be utilized to examine their association even if they may not exactly replicate oral health problems. Additionally, since diabetes can impair mental health, including cognitive function, and since animal models are being used to examine the connection between diabetes and mental health, the field of study is still developing. The models must be modified and adjusted to reflect clinical situations better.
However, it's important to recognise that due to differences in biology and disease progression, a therapy that works well in animal models may not work as well in human clinical trials. Depending on the individual research or drug development demands, the pharmaceutical industry generally choose a variety of diabetic animal models, such as non-human primates, high-fat diet and genetic models, spontaneous diabetic models, and streptozotocin-induced models. Non-human primates pose specific ethical and practical difficulties in terms of housing, expense, and care. These models' direct clinical translation is hampered by a number of constraints, though. These include species differences, the difficulty of replicating complicated human diseases, ethical and practical issues, and the difficulty of accurately predicting human treatment responses. As a result, clinical trials are eventually relied upon to provide important insights into human diabetes and its therapies. Researchers must be aware of these limitations and take them into account when evaluating data from animal studies. It may be challenging to choose viable therapeutic candidates for clinical trials since some animal models' ability to anticipate how humans will react to drugs may be restricted. Nevertheless, despite these limitations, animal models have contributed significantly to our understanding of the pathogenesis of diabetes.
Ethics statements
Not applicable.
CRediT authorship contribution statement
Durga Nandini Athmuri: Conceptualization, Methodology, Writing – original draft. Parvaiz Ahmad Shiekh: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the DST INSPIRE Faculty Fellowship, Govt of India, to Parvaiz Ahmad Shiekh for funding this research.
Data availability
No data was used for the research described in the article.
References
- 1.Sarwar N., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett R. Type 1 diabetes. Lancet. 2018;391:195. doi: 10.1016/S0140-6736(18)30024-2. [DOI] [PubMed] [Google Scholar]
- 3.Rewers M., Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndisang J.F., Vannacci A., Rastogi S. Insulin resistance, type 1 and type 2 diabetes, and related complications 2017. J. Diabetes Res. 2017;2017 doi: 10.1155/2017/1478294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiekh P.A., Singh A., Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249 doi: 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]
- 7.Singh A., Raghav A., Shiekh P.A., Kumar A. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact. Mater. 2021;6:2231–2249. doi: 10.1016/j.bioactmat.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P., Garg A., Garg S., Singh V. Animal model used for experimental study of diabetes mellitus: an overview. Asian J. Biomater. Res. 2016;2:99–110. [Google Scholar]
- 9.Bryda E.C. The mighty mouse: the impact of rodents on advances in biomedical research. Mol. Med. 2013;110:207. [PMC free article] [PubMed] [Google Scholar]
- 10.Heydemann A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannaccone P.M., Jacob H.J. Rats! Dis. Model. Mech. 2009;2:206–210. doi: 10.1242/dmm.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham M.L., Schuurman H.-J. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur. J. Pharmacol. 2015;759:221–230. doi: 10.1016/j.ejphar.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 13.Perlman R.L. Mouse models of human disease an evolutionary perspective. Evol. Med. Public Health. 2016;2016:170–176. doi: 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eleazu C.O., Eleazu K.C., Chukwuma S., Essien U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013;12:1–7. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer T.C., Wilson S.B., Mathews C.E. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010;39:541–561. doi: 10.1016/j.ecl.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis T.M., Atkinson M.A. The clinical significance of an autoimmune response against glutamic acid decarboxylase. Nat. Med. 1996;2:148–153. doi: 10.1038/nm0296-148. [DOI] [PubMed] [Google Scholar]
- 17.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015;70:5.47.1–5.47.20. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 18.Dufrane D., et al. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and β-cell plasticity. Transplantation. 2006;81:36–38. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues B., et al. Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes. 1997;46:1346–1353. doi: 10.2337/diab.46.8.1346. [DOI] [PubMed] [Google Scholar]
- 20.Nelson R.W., Reusch C.E. Animal models of disease: classification and etiology of diabetes in dogs and cats. J. Endocrinol. 2014;222:T1–T9. doi: 10.1530/JOE-14-0202. [DOI] [PubMed] [Google Scholar]
- 21.Rohilla A., Ali S. Alloxan induced diabetes: mechanisms and effects. Int. J. Res. Pharm. Biomed. Sci. 2012;3:819–823. [Google Scholar]
- 22.Nerup J., et al. On the pathogenesis of IDDM. Diabetologia. 1994;37:S82–S89. doi: 10.1007/BF00400830. [DOI] [PubMed] [Google Scholar]
- 23.im Walde S.S., Dohle C., Schott-Ohly P., Gleichmann H. Molecular target structures in alloxan-induced diabetes in mice. Life Sci. 2002;71:1681–1694. doi: 10.1016/s0024-3205(02)01918-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.-R., et al. Role of Ca2+ in alloxan-induced pancreatic β-cell damage. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1994;1227:87–91. doi: 10.1016/0925-4439(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 25.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 26.Wang J., et al. Creating a long-term diabetic rabbit model. Exp. Diabetes Res. 2010;2010:289614. doi: 10.1155/2010/289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y., Santamaria P. Dissecting autoimmune diabetes through genetic manipulation of non-obese diabetic mice. Diabetologia. 2003;46:1447–1464. doi: 10.1007/s00125-003-1218-1. [DOI] [PubMed] [Google Scholar]
- 28.Lenzen S., et al. The LEW. 1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44:1189–1196. doi: 10.1007/s001250100625. [DOI] [PubMed] [Google Scholar]
- 29.Makino S., et al. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 30.Leiter E.H. The NOD mouse: a model for analyzing the interplay between heredity and environment in development of autoimmune disease. ILAR J. 1993;35:4–14. [Google Scholar]
- 31.Hanafusa T., et al. The NOD mouse. Diabetes Res. Clin. Pract. 1994;24:S307–S311. doi: 10.1016/0168-8227(94)90267-4. Suppl. [DOI] [PubMed] [Google Scholar]
- 32.Roep B.O. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann. N. Y. Acad. Sci. 2007;1103:1–10. doi: 10.1196/annals.1394.018. [DOI] [PubMed] [Google Scholar]
- 33.Mordes J.P., Bortell R., Blankenhorn E.P., Rossini A.A., Greiner D.L. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004;45:278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 34.Wallis R.H., et al. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes. 2009;58:1007–1017. doi: 10.2337/db08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss H., et al. Genetic analysis of the LEW.1AR1-iddm rat: an animal model for spontaneous diabetes mellitus. Mamm. Genome. 2005;16:432–441. doi: 10.1007/s00335-004-3022-8. [DOI] [PubMed] [Google Scholar]
- 36.Mathews C.E. Utility of murine models for the study of spontaneous autoimmune type 1 diabetes. Pediatr. Diabetes. 2005;6:165–177. doi: 10.1111/j.1399-543X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 37.Drel V.R., et al. Poly (ADP-ribose) polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int. J. Mol. Med. 2011;28:629–635. doi: 10.3892/ijmm.2011.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou C., Pridgen B., King N., Xu J., Breslow J.L. Hyperglycemic Ins2AkitaLdlr−/− mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease [S] J. Lipid Res. 2011;52:1483–1493. doi: 10.1194/jlr.M014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon J.W., Austin M., Onodera T., Notkins A.L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 40.Utsugi T., et al. A new animal model of non-insulin-dependent diabetes mellitus induced by the NDK25 variant of encephalomyocarditis virus. Diabetes Res. 1992;20:109–119. [PubMed] [Google Scholar]
- 41.Guberski D.L., et al. Induction of type I diabetes by Kilham’s rat virus in diabetes-resistant BB/WOR rats. Science. 1991;254:1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 42.Morel P., et al. Total pancreatectomy in the pig for islet transplantation. Technical alternatives. Transplantation. 1991;52:11–15. doi: 10.1097/00007890-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Fisher S.J., et al. Low-dose IGF-I has no selective advantage over insulin in regulating glucose metabolism in hyperglycemic depancreatized dogs. J. Endocrinol. 2001;168:49–58. doi: 10.1677/joe.0.1680049. [DOI] [PubMed] [Google Scholar]
- 44.He S., et al. Treatment and risk factor analysis of hypoglycemia in diabetic rhesus monkeys. Exp. Biol. Med. 2011;236:212–218. doi: 10.1258/ebm.2010.010208. (Maywood) [DOI] [PubMed] [Google Scholar]
- 45.Wise M.H., Gordon C., Johnson R.W. Intraportal autotransplantation of cryopreserved porcine islets of Langerhans. Cryobiology. 1985;22:359–366. doi: 10.1016/0011-2240(85)90183-x. [DOI] [PubMed] [Google Scholar]
- 46.Boullion R.D., et al. Porcine model of diabetic dyslipidemia: insulin and feed algorithms for mimicking diabetes mellitus in humans. Comput. Med. 2003;53:42–52. [PubMed] [Google Scholar]
- 47.Zhang X., et al. Silkworms can be used as an animal model to screen and evaluate gouty therapeutic drugs. J. Insect Sci. 2012;12:4. doi: 10.1673/031.012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teame T., et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. Rev. Mag. Anim. Agric. 2019;9:68–77. doi: 10.1093/af/vfz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindström P. The physiology of obese-hyperglycemic mice [ob/ob mice] Sci. World J. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chehab F.F., Lim M.E., Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 51.Coleman D.L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 52.Chen H., et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J. Med. Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 54.Phillips M.S., et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 55.Pick A., et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 56.Shibata T., et al. Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonist, JTT-501, on diabetic complications in Zucker diabetic fatty rats. Br. J. Pharmacol. 2000;130:495–504. doi: 10.1038/sj.bjp.0703328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clee S.M., Attie A.D. The genetic landscape of type 2 diabetes in mice. Endocr. Rev. 2007;28:48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 58.Kawano K., Hirashima T., Mori S., Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res. Clin. Pract. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. Suppl. [DOI] [PubMed] [Google Scholar]
- 59.Leiter E.H., Reifsnyder P.C. Differential levels of diabetogenic stress in two new mouse models of obesity and type 2 diabetes. Diabetes. 2004;53(Suppl 1):S4–11. doi: 10.2337/diabetes.53.2007.s4. [DOI] [PubMed] [Google Scholar]
- 60.Kim J.H., et al. Type 2 diabetes mouse model TallyHo carries an obesity gene on chromosome 6 that exaggerates dietary obesity. Physiol. Genomics. 2005;22:171–181. doi: 10.1152/physiolgenomics.00197.2004. [DOI] [PubMed] [Google Scholar]
- 61.Leiter E.H. Selecting the ‘right’ mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol. Biol. 2009;560:1–17. doi: 10.1007/978-1-59745-448-3_1. [DOI] [PubMed] [Google Scholar]
- 62.Cho Y.-R., et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;293:E327–E336. doi: 10.1152/ajpendo.00376.2006. [DOI] [PubMed] [Google Scholar]
- 63.Surwit R.S., Kuhn C.M., Cochrane C., McCubbin J.A., Feinglos M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 64.Winzell M.S., Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 65.Ratiu J.J., et al. Genetic and small molecule disruption of the AID/RAD51 axis similarly protects nonobese diabetic mice from type 1 diabetes through expansion of regulatory B lymphocytes. J. Immunol. 2017;198:4255–4267. doi: 10.4049/jimmunol.1700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burcelin R., Serino M., Chabo C., Blasco-Baque V., Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol. 2011;48:257–273. doi: 10.1007/s00592-011-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearson J.A., Agriantonis A., Wong F.S., Wen L. Modulation of the immune system by the gut microbiota in the development of type 1 diabetes. Hum. Vaccin. Immunother. 2018;14:2580–2596. doi: 10.1080/21645515.2018.1514354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greiner D.L., et al. Humanized mice for the study of type 1 and type 2 diabetes. Ann. N. Y. Acad. Sci. 2011;1245:55–58. doi: 10.1111/j.1749-6632.2011.06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.