Fig. 3.
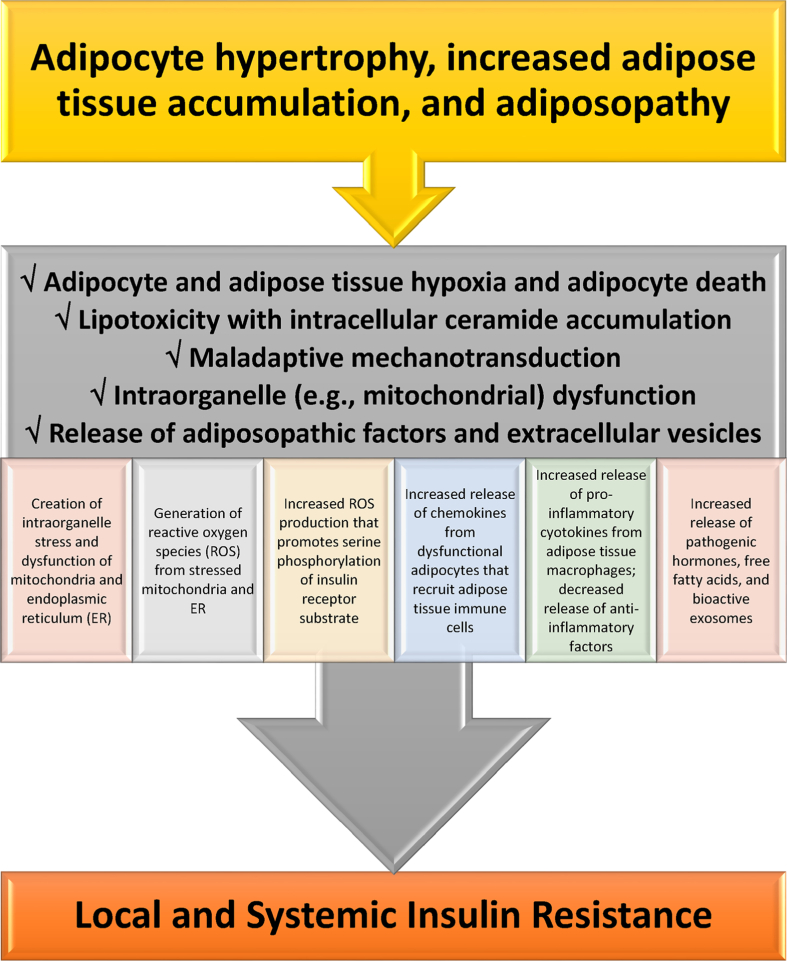
Mechanisms how adiposopathic processes lead to insulin resistance. If obesity-mediated adipocyte hypertrophy and adipose tissue accumulation outgrows vascular supply, then the insufficient delivery of oxygen may contribute to adipocyte and adipose tissue hypoxia and increased adipocyte death. Adipocyte and adipose tissue hypoxia may adversely affect multiple metabolic processes regarding angiogenesis, adipocyte proliferation, adipocyte differentiation, reactive oxygen species generation, inflammation, and fibrosis. Beyond adipocyte and adipose tissue hypoxia, excessive intracellular lipids in the form of fatty acids may lead to ceramide (i.e., a unit of sphingolipids) and diacylglycerol (DAG) formation in adipocytes, where similar to adverse effect of increased fatty acid influx and ceramide and DAG accumulation in liver and muscle, may cause lipotoxicity leading to adipocyte dysfunction [47], such as: (a) inhibiting AKT Protein Kinase B and thus decreasing glucose uptake via GLUT 4, (b) inhibiting hormone sensitive lipase and thus decreasing adrenergic-mediated lipolysis, and (c) impairing mitochondrial function [47], all contributing to insulin resistance. Mechanotransduction occurs when cells sense, integrate, and respond to mechanical stimuli via biologic signaling and adaptations. During healthful expansion, adipose tissue responds by adapting to its microenvironment (e.g., formation, dissolution, and reformation of extracellular matrix) via continuous remodeling to maintain its structural and functional integrity. During positive caloric balance, especially if proliferation is impaired, adipose tissue expansion is often accompanied by hypertrophy of existing adipocytes. Adipocyte hypertrophy, immune cells infiltration, fibrosis and changes in vascular architecture may generate mechanical stress on adipose cells, alter healthful adaptive mechanotransduction, and disrupt healthful adipose cell expansion physiology. Maladaptive mechanotransduction may promote obesity-associated dysfunction in adipose tissue (i.e., adiposopathy) [48]. Overall, contributors to mitochondrial dysfunction include adipocyte and adipose tissue hypoxia, lipotoxicity [47,49], maladaptive mechanotransduction, hyperglycemia [50], and high fat dietary intake [51]. Adipocyte mitochondrial dysfunction is a potential primary cause of adipose tissue inflammation [52]. Among the adverse consequences of adiposopathic mitochondrial (and endoplasmic reticulum) dysfunction is the generation of reactive oxygen species (ROS). ROS are unstable molecules containing oxygen that easily react with other cellular molecules, contributing to deoxynucleic acid damage, cancer, fibrosis, and aging [44]. Other contributors to increased ROS production are hyperglycemia [53] and adiposopathic increases in cytokines such as tumor necrosis factor. Increased tumor necrosis factor-mediated mitochondrial ROS production may facilitate JNK activation, increase serine phosphorylation of insulin receptor substrate-1 (IRS-1), decrease insulin-stimulated tyrosine phosphorylation of IRS-1, and thus contribute to obesity-mediated insulin resistance [54,55]. In summary, adipocyte hypertrophy leading to initial adipocyte dysfunction results in local proinflammatory effects that, in turn, further worsen adipocyte function, resulting in worsening adiposopathy and adipocyte insulin resistance. Systemically, adiposopathic proinflammatory factors, pathogenic hormones, and free fatty acids may be released into the circulation either directly from adipose tissue, or via adipocyte extracellular vesicles (e.g., bioactive molecules such as lipids, proteins, and nucleic acids that are packaged and transferred from adipocytes to other body tissues via exosomes, microvesicles, and apoptotic bodies formed as the result of adipocyte necroptosis or pyroptosis). The increase in pro-inflammatory factors (e.g., tumor necrosis factor and interleukins 1 beta and 6) [56] and decrease secretion of anti-inflammatory factors (e.g., adiponectin) [57] may promote insulin resistance (i.e., reduced cellular surface insulin receptors and post-insulin receptor defects) in susceptible non-adipose tissue peripheral organs, such as skeletal muscle and liver, contributing to “inflexibility” in managing, responding or adapting to changes in metabolic substrates.