Abstract
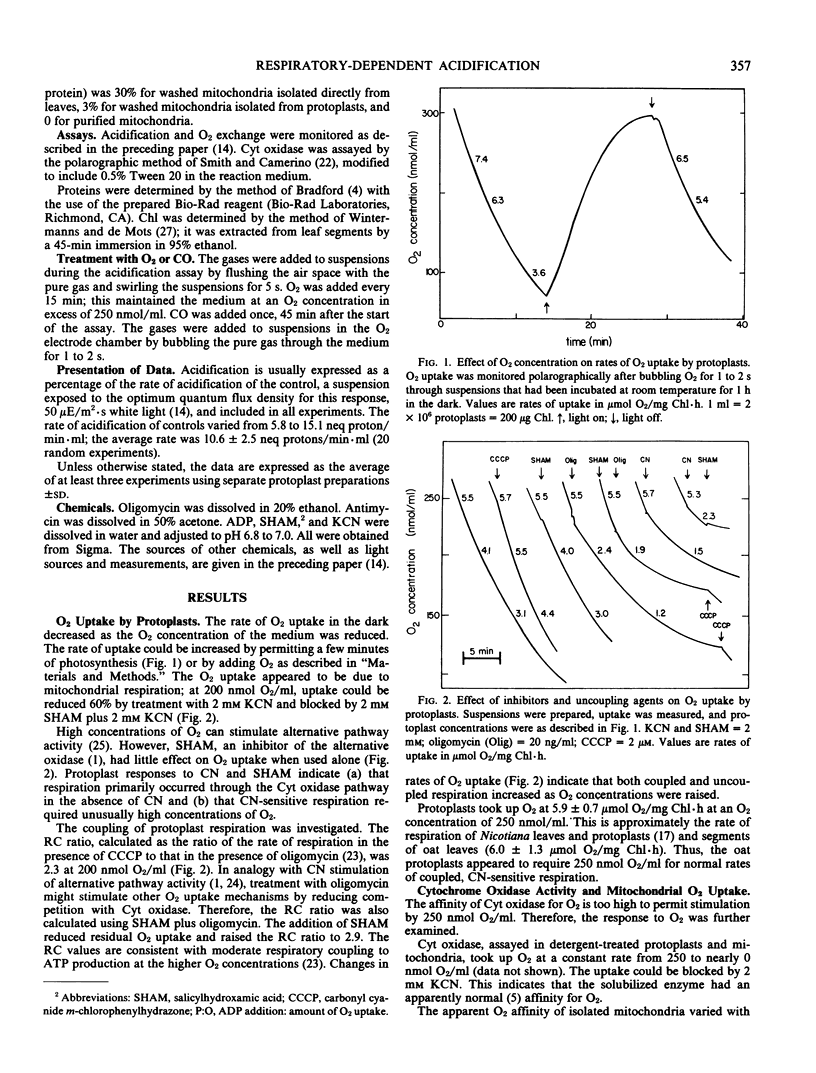
Some photosynthetically stimulated acidification of the medium by oat (Avena sativa L. cv Garry) leaf protoplasts required respiration. The requisite respiration (a) had a low apparent affinity for O2, (b) was blocked by cyanide plus salicylhydroxamic acid, (c) characterized protoplasts and mitochondria isolated from protoplasts, (d) could be induced in leaf segments, and (e) appeared to result from an inhibition of mitochondrial respiration that included the cytochrome pathway.
Carbon monoxide and cyanide prevented acidification of weakly photosynthesizing suspensions. Salicylhydroxamic acid had no effect on acidification, indicating a specific dependence upon cyanide-sensitive respiration. Photosynthesis stimulated acidification through stable products, and exogenously supplied O2 stimulated acidification. The acidification response to O2 was additive to the response to photosynthesis at subsaturating levels of light, indicating a common mode of action. Oligomycin prevented stimulation of acidification by low levels of photosynthetic activity; this stimulation appeared to be due to O2-induced increases in mitochondrial energy production. Oligomycin only partially inhibited stimulation of acidification by higher levels of light; this stimulation appeared to be partially dependent upon photophosphorylation. Therefore, oligomycin-sensitive acidification of the medium appeared to reflect changes in mitochondrial energy production in photosynthesizing protoplasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Behrens P. W., Marsho T. V., Radmer R. J. Photosynthetic o(2) exchange kinetics in isolated soybean cells. Plant Physiol. 1982 Jul;70(1):179–185. doi: 10.1104/pp.70.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chastain C. J., Lafayette P. R., Hanson J. B. Action of protein synthesis inhibitors in blocking electrogenic h efflux from corn roots. Plant Physiol. 1981 Apr;67(4):832–835. doi: 10.1104/pp.67.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G., Gerola P. Inhibition of photosynthesis by azide and cyanide and the role of oxygen in photosynthesis. Plant Physiol. 1977 May;59(5):859–862. doi: 10.1104/pp.59.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S. Light-induced h secretion and the relation to senescence of oat leaves. Plant Physiol. 1982 Oct;70(4):1120–1124. doi: 10.1104/pp.70.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., André M. Effect of CO(2), O(2), and Light on Photosynthesis and Photorespiration in Wheat. Plant Physiol. 1980 Dec;66(6):1032–1036. doi: 10.1104/pp.66.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Light-Stimulated Changes in the Acidity of Suspensions of Oat Protoplasts: Dependence upon Photosynthesis. Plant Physiol. 1983 Jun;72(2):351–355. doi: 10.1104/pp.72.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Douce R., Akazawa T. Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol. 1982 Apr;69(4):916–920. doi: 10.1104/pp.69.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L., CAMERINO P. W. COMPARISON OF POLAROGRAPHIC AND SPECTROPHOTOMETRIC ASSAYS FOR CYTOCHROME C OXIDASE ACTIVITY. Biochemistry. 1963 Nov-Dec;2:1428–1432. doi: 10.1021/bi00906a039. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Respiration and the intracellular potential. J Gen Physiol. 1965 Sep;49(1):93–116. doi: 10.1085/jgp.49.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Selective enhancement of alternative path capacity in plant storage organs in response to ethylene plus oxygen: a comparative study. Plant Physiol. 1982 May;69(5):1036–1039. doi: 10.1104/pp.69.5.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Earle E. D., Yoder O. C., Spanswick R. M. Reduction of Adenosine Triphosphate Levels in Susceptible Maize Mesophyll Protoplasts by Helminthosporium maydis Race T Toxin. Plant Physiol. 1979 May;63(5):806–810. doi: 10.1104/pp.63.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]


