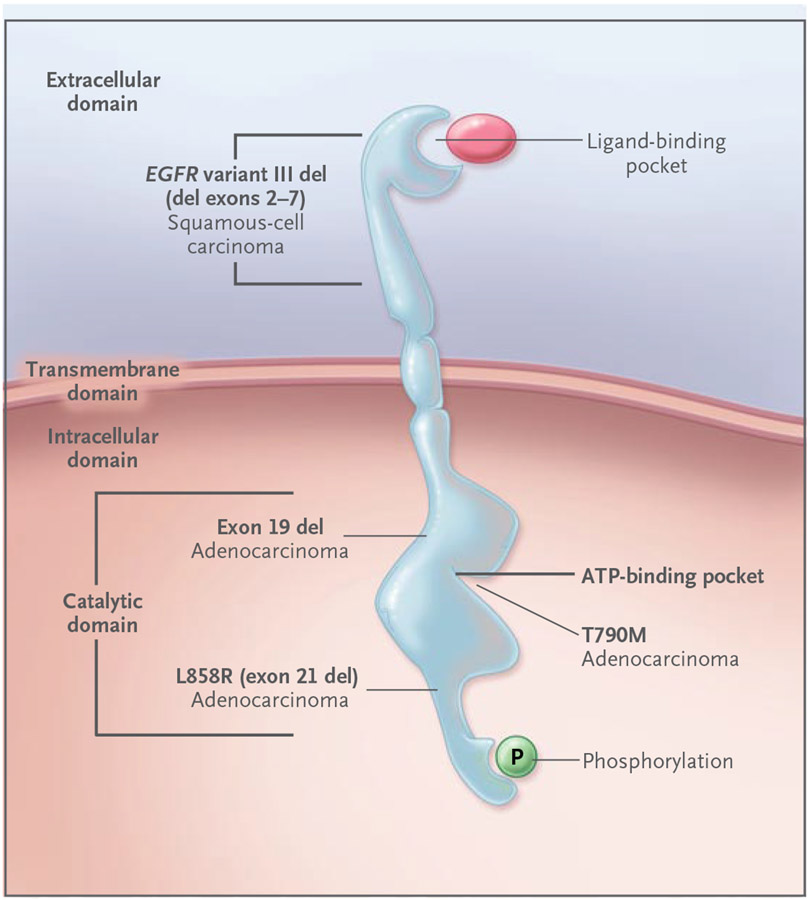
Figure 3. Effect of Deletions and Mutations in the Epidermal Growth Factor Receptor Gene (EGFR) on Disease Development and Drug Targeting.
Ligand binding to the EGFR extracellular domain results in receptor homodimerization and tyrosine phosphorylation, with the phosphate derived from ATP bound within the kinase catalytic domain. EGFR mutations have transforming potential in preclinical lung models and can occur early in human lung carcinogenesis. EGFR point mutations (e.g., L858R) and exon 19 deletions, which occur predominantly in adenocarcinoma of the lung, are located within the catalytic domain and result in constitutive EGFR activation. These mutations are associated with increased sensitivity to EGFR tyrosine kinase inhibitors, such as erlotinib and gefitinib. In contrast, mutations in T790M (an amino acid located within the ATP binding site of the EGFR kinase domain) are associated with acquired resistance to these drugs. EGFR variant III mutant deletions occur in the extracellular domain and are associated with squamous-cell cancer.