Abstract
The role of thalamocortical circuits in memory has driven a recent burst of scholarship, especially in animal models. Investigating this circuitry in humans is more challenging. And yet, the development of new recording and stimulation technologies deployed for clinical indications has created novel opportunities for data collection to elucidate the cognitive roles of thalamic structures. These technologies include stereoelectroencephalography (SEEG), deep brain stimulation (DBS), and responsive neurostimulation (RNS), all of which have been applied to memory-related thalamic regions, specifically for seizure localization and treatment. This review seeks to summarize the existing applications of neuromodulation of the anterior thalamic nuclei (ANT) and highlight several devices and their capabilities that can allow cognitive researchers to design experiments to assay its functionality. Our goal is to introduce to investigators, who may not be familiar with these clinical devices, the capabilities, and limitations of these tools for understanding the neurophysiology of the ANT as it pertains to memory and other behaviors. We also briefly cover the targeting of other thalamic regions including the centromedian (CM) nucleus, dorsomedial (DM) nucleus, and pulvinar, with associated potential avenues of experimentation.
Keywords: Anterior thalamic nuclei, Deep brain stimulation, Neuromodulation, Memory, Responsive neurostimulation, Epilepsy
Graphical abstract
Highlights
-
•
DBS targets the ANT and is effective for refractory temporal lobe epilepsy.
-
•
The ANT is involved in memory and spatial navigation, making it a viable DBS target.
-
•
Thalamic circuitry can be explored via DBS, RNS, and SEEG techniques.
-
•
Neuromodulation of the thalamus could reveal biomarkers for intervention.
-
•
DBS, RNS, and SEEG offer new ways to investigate the ANT and its functions.
1. Introduction
The application of deep brain stimulation (DBS) on the anterior nuclei of the thalamus (ANT) has proven to be an effective treatment option for individuals suffering from drug-resistant epilepsy (DRE). Investigation into this clinical option was driven by its demonstrable efficacy and safety in other disease states and nearby anatomic locations, such as the subthalamic nucleus (STN) and globus pallidus internus in Parkinson's Disease (PD) and the ventral intermediate nucleus of the thalamus in essential tremor (Barbe et al., 2018; Georgiev et al., 2021; Lega et al., 2011; Negida et al., 2018). The effectiveness of this treatment method was first exhibited in the SANTE trial, after which it became an FDA approved treatment for medically intractable epilepsy in 2018 (Fisher et al., 2010; Shafer, 2018).
Following the publication of the SANTE trial, ANT-DBS was extensively incorporated into clinical practice. However, the exact mechanism underlying the clinical effectiveness of this therapy and the potential effects of long-term stimulation on cognitive circuits, which involve the ANT, are still not fully understood (Nelson, 2021; Perry and Mitchell, 2019). This knowledge gap underscores the importance of gaining a deeper understanding of the functional circuitry of ANT. Significant progress has been made in the domain of neuromodulation technologies such as DBS, responsive neurostimulation (RNS), and stereoelectroencephalography (SEEG), which offer promising opportunities for researchers to investigate the ANT and its role in human cognitive processes. The aim of this review is to explore these new insights at the intersection of neuromodulation technologies and cognitive experimentation, as it pertains to the ANT.
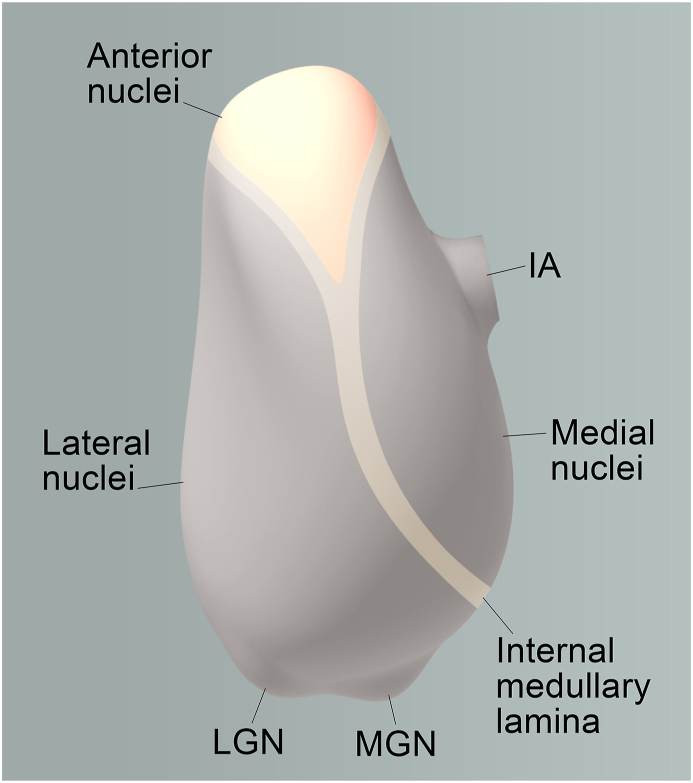
The ANT is found in the superior region of the thalamus (Fig. 1) and can be further classified into three functional nuclei: the anteroventral (AV), anterodorsal (AD), and anteromedial (AM) nuclei (Child and Benarroch, 2013). The AM nucleus is hypothesized to constitute a predominantly feed-forward mechanism that transmits consolidated information from the hippocampal-diencephalic network to the prefrontal regions, thereby participating in high-level cognitive and executive processes. Conversely, the AV nucleus is primarily involved in a feedback mechanism, aimed at maintaining rhythmic theta activity to the hippocampal formation. Lastly, the AD nucleus is believed to host the head direction system, given that its cells demonstrate electrophysiological compass-like traits, responding selectively to head directions, rather than locations. (Aggleton et al., 2010; Clark and Taube, 2012; Jankowski et al., 2013; Taube, 2007).
Fig. 1.
Thalamic nuclei and the anterior thalamic nuclei (ANT) highlighted in yellow.
This image depicts the left thalamus, a key relay center in the brain, with the ANT prominently highlighted in yellow. Abbreviations in the figure include IA (Interthalamic adhesion), MGN (medial geniculate nucleus), and LGN (lateral geniculate nucleus). The ANT is known to play a crucial role in learning and memory processes, as well as in spatial navigation. These findings underscore the importance of the thalamus, and the ANT in particular, in a variety of brain functions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Located within a complex network of connections, the ANT interacts with both cortical and subcortical regions (Child and Benarroch, 2013). It exhibits a greater number of connections with the cingulate cortex and temporal lobes, specifically the hippocampus, than with the frontal lobes, where its connections are confined to the AM and AV nuclei (Amaral and Cowan, 1980; Hicks and Huerta, 1991; Jankowski et al., 2013; Van Groen and Wyss, 1995). Furthermore, the ANT receives dense inputs from the retrosplenial cortex, the subiculum, and the mammillary bodies (via the mammillothalamic tract) (Jankowski et al., 2013; Wright et al., 2010). We will show that modern technologies allow for better understanding of these circuits and their functional significance.
2. The current understanding of the ANT: Lessons from intracranial recording and neuromodulation
2.1. Stimulation of the ANT for drug resistant epilepsy
The ANT displays a high level of connectivity with brain regions typically associated with temporal lobe and “temporal lobe plus” epilepsy (Aggleton and Brown, 1999; Aggleton and O'Mara, 2022; Aggleton et al., 2010; Jankowski et al., 2013). Based on data from experimental models and anatomical insights regarding major projections of the ANT to the neocortex and limbic structures, several pilot studies postulated that targeting the ANT would be more effective in desynchronizing widespread cortical areas than other, previously tested thalamic nuclei (Cooper et al., 1984; Fisher et al., 2010; Kerrigan et al., 2004). These studies laid the groundwork for the SANTE clinical trial.
The SANTE trial was a multicenter, double blinded, randomized controlled trial that evaluated the effect of direct stimulation of the ANT on seizure frequency and severity in patients with DRE. In patients for whom it was determined that neurosurgical resection would not be of benefit, an implanted neuromodulatory device was offered. During the trial, 110 patients between the ages of 18 and 65 who experienced both partial and generalized seizures were enrolled and received bilateral stereotactic implantation of electrodes in the ANT. The trial utilized LEAD-DBS software (Horn and Kühn, 2015) to localize DBS leads in relation to the ANT. The contact closest to the ANT center was used as the cathode in monopolar stimulation with the case set as the anode (voltage = 5 V, frequency = 145 Hz, pulse width = 90 μs, 1 min ON/5 min OFF) (Lega et al., 2010). Results from the study reported that in the last month of the blinded phase, the group receiving stimulation had a 29% larger reduction in seizures than the control group (Fisher et al., 2010). At the end of 2 years, there was a reported 56% reduction in seizure frequency; 54% of patients had a seizure reduction of at least 50%, as well as 14 patients who reported being seizure-free for 6 months or more.
These results provided evidence that bilateral ANT-DBS could offer substantial clinical benefit in cases of focal epilepsy, particularly temporal lobe epilepsies. As a result, it was granted FDA approval for DRE in 2018 (Fisher et al., 2010; Shafer, 2018). A long-term follow-up of 13 months post-SANTE trial and onwards showed sustained efficacy of ANT-DBS (Salanova et al., 2015).
It is crucial to mention that patients who are most likely to benefit from this treatment, as observed in the SANTE trial, are those who suffer from severe epilepsy, often affecting the thalamic area. Additionally, because this intervention is mainly studied in patients with severe epilepsy, investigators should consider patient and disease-specific contributions to aberrant neurophysiology when interpreting their results. This limitation is not limited to thalamic or epilepsy studies but applies to most investigations into human neurophysiology that utilize recordings from devices initially placed to treat local and distributed pathologies.
2.2. Long-term effects of ANT-DBS on memory
The long-term outcomes of ANT-DBS on memory and mood were analyzed in a follow-up of the SANTE clinical trial (Tröster et al., 2017). Seven years after its initiation, subjective reports of both depression and memory adverse events (AEs) were more associated with the implanted and stimulated group, compared to the implanted and non-stimulated (control) group (Tröster et al., 2017). However, the same patients underwent an objective neurobehavioral evaluation that included testing of visuospatial memory, language, executive function, subjective cognitive function, behavior disturbance, processing speed, and depression; the results showed no differences between the stimulated and non-stimulated groups (Tröster et al., 2017). The researchers discussed possible reasons for the incongruity between reported AEs and objective measures and concluded that the evaluation tools were perhaps not specific enough to elucidate a clear result (Tröster et al., 2017). Furthermore, it is possible subjects experiencing a reduction in seizures and improved cognition are more aware of their cognitive deficits. This is supplemented by the fact that those complaining of memory AEs had an improved measured verbal memory score (Tröster et al., 2017). Additional findings showed that six of the eight subjects reporting depression AEs in the blinded phase of the trial had been diagnosed with depression prior to the study. Moreover, the neuropsychiatric changes resulting from ANT-DBS may have also been from the residual effects of stimulation, such as disruption of sleep. Though their overall results were inconclusive, findings suggest neurobehavioral evaluation should be regularly administered before, during, and after ANT-DBS (Tröster et al., 2017). Investigators interested in patient-reported experiences with these devices as they pertain to memory or other neurocognitive functions should consider these findings when interpreting their results.
Another study examining memory skills one year after ANT-DBS reported improvements in verbal information processing and recall, although it is difficult to disambiguate these findings from the beneficial impact of DBS on seizure frequency. The authors suggested this may be a result of fronto-limbic circuitry activation, caused by the DBS surgery (Oh et al., 2012).
In summary, there are few publications that assess the chronic impact of ANT stimulation on memory performance, and their conclusions may be limited. Similarly, specific investigations into the effects of long-term stimulation on distinct paradigms such as episodic versus working memory have not been reported. This represents an important gap in current knowledge related to ANT function.
2.3. Episodic and autobiographical memory
In humans, bilateral lesions to the medial diencephalon are responsible for diencephalic amnesia, which closely resembles temporal lobe amnesia (Aggleton, 2008; Kopelman et al., 1995). Specifically, damage to the ANT from excessive alcohol use (Korsakoff's Syndrome), neurodegenerative disorders, and thalamic strokes have been observed to impair episodic memory formation (Harding et al., 2000; Segobin et al., 2019; Van der Werf et al., 2003). Though the origins of diencephalic amnesia are rather complex, lesions to the ANT appear to be a primary driver for the disease state, proposing its role in the episodic memory network (Carlesimo et al., 2011; Frost et al., 2021; Harding et al., 2000; Segobin et al., 2019; Van der Werf et al., 2003).
In patients with DRE and ANT-DBS, event-related potentials (ERPs) elicited during visual and verbal memory tasks revealed that in this memory circuit, the ANT processes information before it is conveyed to the hippocampus, signifying a potential role in memory recognition processes (Štillová et al., 2015) that may include cue specification or attentional selection of extrinsic information to drive novel memory formation. Moreover, the proposed similarity between the circuitry involved in spatial and episodic memory implies that the ANT plays a similar role in processing episodic memories (Štillová et al., 2015).
The use of intracranial electrophysiological recordings has enabled researchers to investigate the function of the ANT during memory behavior. Sweeney-Reed et al. review the findings of multiple different recordings across a series of studies (Sweeney-Reed et al., 2014, 2016, 2021; Sweeney-Reed et al., 2016a, Sweeney-Reed et al., 2016b). Investigators measured synchrony between the human ANT and the neocortex during successful memory formation along with complementary tasks, in a total of 11 patients. These patients engaged in various cognitive paradigms including subsequent memory, novelty oddball, and recognition memory (Sweeney-Reed et al., 2021). During memory encoding performance, recordings revealed synchronization of theta frequencies between 5 and 6 Hz between the ANT and the frontal and parietal cortex approximately 1 s post-stimulus in cases of successful memory encoding (measured with concomitant surface EEG) (Sweeney-Reed et al., 2014). In another study involving the ANT during mnemonic function (Zotev et al., 2018), participants were asked to retrieve an autobiographical memory while undergoing functional MRI (fMRI). ANT BOLD activity was coherent with EEG-measured alpha activity. The experimental group learned to upregulate BOLD activity of the target region (the MD and ANT) using real-time fMRI neurofeedback, which resulted in enhanced temporal correlation between thalamic BOLD activity and EEG alpha power (Zotev et al., 2018).
Using intrathalamically implanted electrodes, researchers can reveal modulatory interactions amongst the thalamic nuclei, such as recording from both MD and ANT nuclei. While this is currently a rare phenomenon, we anticipate that such cases will emerge as an increased volume of ANT-DBS reveals a subset of non-responding patients; this may provide an opportunity to investigate these relationships. Several theories have been proposed regarding the correlative functions of the ANT and MD (Aggleton, 2012; Aggleton et al., 2010; Carlesimo et al., 2011; Edelstyn et al., 2002; Van der Werf et al., 2003). In one study by Sweeney-Reed et al., four-contact electrodes were placed in the bilateral ANT and MD of patients who underwent a memory encoding test (Sweeney-Reed et al., 2016a, Sweeney-Reed et al., 2016b). The group discovered that higher pre-stimulus MD theta power predicted encoding success. Additionally, they reported that pre-stimulus theta power in the MD predicted post-stimulus correlates of successful memory formation in the ANT.
Furthermore, an examination of interictal epileptiform discharges (IEDs) discovered variations in IED rates between the ANT and the MD, indicating the participation of both thalamic nuclei in distinct epileptic networks that vary across individual patients (Sweeney-Reed et al., 2016a, Sweeney-Reed et al., 2016b). In summary, the use of intrathalamic electrodes has provided valuable insights into the modulatory interactions between thalamic nuclei, and future research may further enhance our understanding of these relationships.
2.4. Working memory
Recent research has identified a significant involvement of the ANT in working memory (Liu et al., 2021; Roy et al., 2022). In a study utilizing a working memory task, bipolar stimulation of the ANT was observed to significantly enhance working memory precision relative to participants who underwent implantation of electrodes but were not subjected to stimulation. The randomly selected half of participants who received stimulation received biphasic rectangular pulses with a width of 300 μs and an amplitude of 0.2 mA at a frequency of 50 Hz to paired neighbor contacts within the ANT. In this same study, researchers also found that ANT stimulation increased hippocampal gamma power and decreased IED occurrence rate. This increase in gamma power was found to be highly correlated with improved working memory precision, highlighting the impact of the hippocampal-anterior thalamic axis on the medial temporal lobe and working memory (Liu et al., 2021).
These models assume that the hippocampus plays a causal role in working memory, which is not canonically accepted, and alternative proposals include engagement of prefrontal regions with dense ANT connectivity. It is known that the hippocampus has direct links to the prefrontal cortex, and manipulations to the hippocampus have been demonstrated to affect working memory across several models (Wirt and Hyman, 2017). Meanwhile, recent animal models have shown that manipulation of the ANT results in a much wider range of cognitive deficits (Bubb et al., 2021).
Regardless of underlying mechanism, ANT high frequency stimulation elicits an increase in hippocampal gamma power, a response that predicted increased accuracy in working memory (Liu et al., 2021). These findings further support that ANT stimulation leads to a widespread impact on frontal circuits (Middlebrooks et al., 2020; Nelson, 2021), although this idea contradicts the specific functional association seen in context-dependent mnemonic processing. Dissecting the potential contributions of distinct ANT subregions may require testing patients with slightly different implantation locations, as this can be somewhat variable across individuals. Some precedence for such analyses exists in DBS applied for movement disorders, in which affective versus movement related STN subnuclei have been determined using slightly varied implantation locations across larger sets of patients (Negida et al., 2018; Rizzone et al., 2001; Su et al., 2019).
2.5. Spatial navigation
Due to its well-investigated connections to hippocampus and entorhinal cortex, as well as the availability of experimental models that analogize episodic memory in humans with spatial navigation in animals, a large body of work has come to support an integral role for the ANT in spatial navigation (Jankowski et al., 2013). This section will serve to outline this role in the context of the specific cell populations found in the ANT and its subnuclei.
It is important to place the role of the ANT in spatial processing in the context of the larger system in which it is a part. Key structures within this system include specific cell populations within the hippocampus and entorhinal cortex that integrate and record allo- and ego-centric information regarding one's environment to aid both in navigation and in memory. Specifically, place cells refer to hippocampal neurons that consistently represent specific locations in a spatial environment; readers are directed to the extensive work of Dr. John O'Keefe and colleagues for in-depth review of their methods and discovery (O'Keefe et al., 1998; O'Keefe and Dostrovsky, 1971). Similarly, and important to our discussion of these data in episodic memory, hippocampal time cells provide representations of temporal contextual information, as articulated by Eichenbaum and others (Buzsáki and Tingley, 2018; Eichenbaum, 2014, 2017; Umbach et al., 2020). These place and time cells are informed by analogs in the entorhinal cortex referred to as grid and ramping cells, respectively, and serve to integrate and relay sensory information from the cortex to the hippocampus (Grossberg and Pilly, 2014; Umbach et al., 2020). These data are complemented by information from the ANT generated by a population of neurons termed “head direction (HD) cells”. In animal studies, HD cells have been shown to furnish rats with the essential information to successfully navigate spatial problems intelligently and proficiently, firing robustly upon head turning to support creation of a cognitive map of one's environment (Muller, 1996, Taube, 2007). These cells are the principal mnemonically related cell populations observed in the ANT in rodent models, and exhibit firing rate changes that are sensitive to egocentric spatial perspective (Gibson, 2013, Muller, 1996, Taube, 2007).
Recordings of HD cells within the AD nucleus of the ANT show a unimodal projection from the AD to its main cortical target, the post-subiculum, via the retrosplenial cortex. The activity and information flow in this network remain unchanged upon varying brain states (static, temporal, or inter-area) (Chaitanya et al., 2020; Clark and Taube, 2012; Peyrache et al., 2019; Peyrache et al., 2019). During sleep, HD cell inputs are suggested to participate in hippocampal replay and assist in the consolidation of memory (Peyrache et al., 2019), potentially by acting as a relay between the hippocampus, medial prefrontal cortex, and posterior representational areas. These findings suggest that neurons in the HD network are fundamentally driven by intrinsic factors, rather than by sensory input, contributing to bottom-up information flow (Peyrache et al., 2019; Peyrache et al., 2019).
One proposed mechanism by which the egocentric information from HD cells is incorporated into the distributed spatial processing apparatus is via integration through theta activity. In response to an applied theta rhythm, all three anterior thalamic nuclei contain “theta-on” cells that respond. However, single-unit recordings in urethane-anesthetized rats have shown that only the AV subnucleus independently fires in theta rhythm (Vertes et al., 2001). The AV displays extensive interaction with the subiculum, and retrosplenial cortex anterior cingulate cortex and the secondary motor cortex (Child and Benarroch, 2013). An estimated 75% of AV neurons display slow and fast burst spiking selectively in theta rhythm (Vertes et al., 2001), with a significant proportion (39%) of HD cells in the AV demonstrating rhythmic spiking in the theta range (Tsanov et al., 2011; Vertes et al., 2001). This latter cohort of cells are referred to as “head-direction-by-theta” cells, and their discovery provides evidence that the integration of head-directional and theta activity occurs at the level of the AV subnucleus of the ANT (Jankowski et al., 2013; Tsanov et al., 2011). Notably, these spatially sensitive cell populations in the ANT have not yet been investigated or verified in humans. While current neuromodulation devices lack the resolution to record from these neurons individually, investigators may be interested in utilizing their precisely timed disruptive stimulation and/or the recording of local field potentials (LFPs) to examine the role of the ANT in spatial navigation within human subjects.
Lastly, animal models have also shown a unique relationship between the pathways of the ANT and the vestibular sensory system (Muir et al., 2009). Angular velocity information from the vestibular system to thalamic nuclei is used by the ANT to output what is known as an ‘absolute head direction signal’(Muir et al., 2009; Sharp et al., 2001; Taube, 2007). This signal facilitates the representation of position and influences the production of cognitive and spatial maps in cortical areas such as the post-subiculum, medial entorhinal cortex and hippocampus (Peyrache et al., 2019).
While further investigation is necessary, the discovery of HD cells and their extensive connections, as well as input from local theta activity and the vestibular system support the notion that the ANT plays a critical role in spatial processing.
3. ANT targeting and stimulation parameters for DBS
The most common clinical indication for neuromodulatory targeting of the ANT is DBS for patients with DRE. During this procedure, between one and four electrode contacts are placed stereotactically into bilateral ANT. These electrodes are connected to a subcutaneously implanted generator, usually in the chest of the patient, that supplies the current.
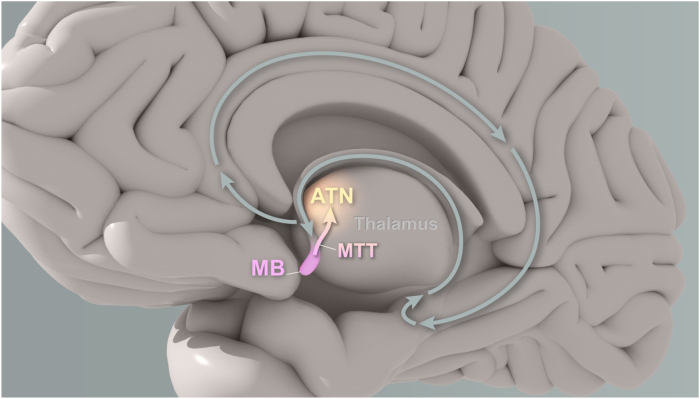
Interpatient anatomic variability can make targeting the ANT challenging, and local landmarks are often employed to guide placement (Möttönen et al., 2015). One such landmark are the mammillary bodies. These small, round, diencephalic structures sit at the base of the brain and project unimodally to the ANT via the mammillothalamic tract (MTT) (Fig. 2); this pathway is important for recollective memory (Jankowski et al., 2013; Peterson et al., 2019). A comparison of 3T MRI short tau inversion recovery (STIR) images found the MTT to be the most clearly visualized structure around the ANT; thus, acting as a key landmark during MRI-guided targeting of the ANT (Balak et al., 2018; Möttönen et al., 2015). Additionally, emerging consensus has explained that optimal clinical targeting of the ANT during neuromodulation utilizes the MTT because therapeutic stimulation effect requires stimulation of white matter fibers within this region (Freund et al., 2022; Ilyas et al., 2022; Koeppen et al., 2019).
Fig. 2.
Projection of the MTT from the MB to the ANT.
The figure shows a sagittal view of the brain, with the small, round, diencephalic mammillary bodies (MB) located underneath. The unimodal projection of the mammillothalamic tract (MTT) from the MB to the anterior thalamic nuclei (ANT) is highlighted. This pathway is known to play a crucial role in recollective memory.
Lehtimaki et al. sought to identify clinical outcome (measured as reduction in seizures) in regard to contact placement within the ANT and reported that leads placed more anteriorly and more superiorly provided a greater therapeutic effect (Lehtimäki et al., 2016). Once implanted, the only modifiable factor of the DBS system is the programming of the pulse generator. The typical DBS programming parameters are frequency, ON/OFF settings, pulse-width, stimulation amplitude, and polarity (Ramasubbu et al., 2018).
How best to adjust these parameters of stimulation to get a desired effect is an empirical question specific to a particular experiment. Published stimulation protocols often differ depending on the study as well as the objective of the stimulation. In this section, we briefly review some of the evidence for each of these parameters to provide investigators with a primer on their use.
3.1. Frequency
Frequency parameters vary depending on the intended electrophysiological outcome. In the thalamus, for example, high frequency stimulation (100-330 Hz) causes a short calcium spike in neurons with bursting firing pattern, followed by activity inhibition of many thalamic cells that may last for up to 10 s. This process lends itself to the notion that thalamic neurons become hyperpolarized during stimulation, though the mechanism is incompletely understood (Dostrovsky and Lozano, 2002; Su et al., 2019). The acute versus chronic effects of stimulation remain largely unknown in terms of physiological impacts, although there is a consensus that high frequency stimulation attenuates local activity in each region. In previous investigations, perturbations of cognitive circuits with high frequency DBS have been interpreted as inducing a temporary lesion at the stimulation site (Benazzouz and Hallett, 2000; Frank et al., 2007). In clinical applications, the effects of frequency, such as high frequency versus low frequency stimulation, have been found to create dramatic differences in PD patients with chronic pain, with low frequency stimulation being preferred due to its greater effect on thermal and mechanical detection (Belasen et al., 2017). Additionally, when studying patients with treatment-resistant depression, frequency of stimulation was regarded as the second-most significant contributor to DBS outcome, the first being the contact configuration of the DBS leads (Sheth et al., 2022). Investigators considering stimulation experiments will have to carefully compare frequency parameters that have been published in their region of interest and experimental paradigm to best inform their design.
3.2. ON/OFF settings and feedback
Time spent in an ON or OFF stimulation state can be modified for an individual patient based on their clinical response. Most current DBS systems utilize “open-loop” systems by delivering continuous stimulation through the manually set, pre-programmed ON/OFF periods, managed by their treating physician. These settings can be adjusted at follow-ups, based on their symptomatic response to treatment (Fasano et al., 2012; Schlaepfer et al., 2013). While generally successful, these systems do not incorporate variations in brain activity, and thus, for complex cognitive neuromodulation closed-loop systems may be more efficacious.
DBS closed-loop feedback systems continuously monitor patients' neural activity and adjust stimulation accordingly. To create a closed-loop DBS system, it is essential to first identify an easily measurable electrophysiological signal that provides predictive information concerning a patient's pathology to use as a trigger for stimulation. Additionally, a feedback signal can be obtained by measuring the neuronal brain activity in response to stimulation. Either of these signals could, in theory, be generated by individual neuronal activity at the implant site or from LFPs, but investigators will be limited by the recording capability of their chosen device and the precision of lead placement. Planning experiments with these interventions will thus require close communication with the device provider and with the clinical team dictating placement.
3.3. Pulse-width
In DBS, pulse-width, or pulse duration, generally tends to be of short duration, such as 60–100 μs (Ramasubbu et al., 2018). Studies of essential tremor investigating thalamic stimulation have reported long pulse-widths as being associated with cognitive deficits (Woods et al., 2003). Meanwhile, short pulse-widths have been demonstrated to produce a greater therapeutic window for voltage adjustments while minimizing charge, and thereby damage, to the targeted region (Gorman and Mortimer, 1983; Ramasubbu et al., 2018; Rizzone et al., 2001). The precise location of lead placement can often dictate the response to pulse width. For example, a study by Anderson et al. demonstrated that stimulation of small, proximal axons are associated with therapeutic benefit, while stimulation of larger, more distant axons are associated with adverse effects (Anderson et al., 2020). Through multicompartmental NEURON models, short pulse-widths were found to demonstrate selectivity for large axons, with long pulse-widths demonstrating selectivity for small, proximal axons (Anderson et al., 2020; Gorman and Mortimer, 1983; Ramasubbu et al., 2018; Rizzone et al., 2001). The detailed effects of pulse-width tuning in DBS may be location and disease-specific and will require further study for clarification.
3.4. Stimulation amplitude and polarity
The stimulation amplitude for either voltage or current can be adjusted to optimize clinical efficacy while minimizing side effects. Generally, the amplitude of current or voltage is increased if a response is clinically insufficient up to a maximum current of approximately 6 milliampere (mA), and voltage of 4 volts (V) (Elsanadidy et al., 2022; Ramasubbu et al., 2018).
For stimulation polarity, there are two common stimulation modes: monopolar and bipolar. In both modes, electrical current flows from anode to the cathode, causing depolarization of the neural elements close to the cathode and hyperpolarization of the neural elements close to the anode. The cathode acts as a negative electric potential, while the anode serves as a positive electric potential, or current source. In the monopolar configuration, the implanted pulse generator acts as the anode, while one or more electrode contacts are designated as the cathode. In contrast, in bipolar stimulation, one electrode contact acts as the anode and another contact serves as the cathode. An important consideration for method of stimulation choice and lead placement is the expected “volume of tissue activated” (VTA). Monopolar stimulation typically stimulates a larger volume of tissue around the cathodal pole, creating a roughly spherical shape, while bipolar stimulation produces an ellipsoid shape around the cathodal contact. Monopolar stimulation is usually preferred as it requires lower intensities to achieve therapeutic benefits, but it can cause more significant side effects due to wider dispersion of the current, often to ‘off target’ impacts outside the target region. In cases where stimulation-related side effects occur, switching from monopolar to bipolar may be a viable solution, provided that reducing the amplitude of the monopolar stimulation fails to address the issue (Ramasubbu et al., 2018). The VTA can be affected by all of the modifiable stimulation parameters and is an important consideration in clinical practice to maximize therapeutic benefit and reduce side effects. Multiple methods for computing and estimating VTA have been published, Duffley et al. (2019) review and evaluate of some of the most common methods.
3.5. Selecting stimulation parameters
Parameter selection remains empirical. Neurologists use external patient programmers to select from a “menu” of typical stimulation profiles, which is guided by post-operative imaging showing the leads that are localized in the preferred region of the ANT. Once selected, these parameters are held in place for a period of weeks to months, and in the case of the ANT, seizure reduction response is recorded. In turn, this measure is used for adjusting stimulation parameter settings at future visits. It should be noted that individual patient responses to neuromodulation vary due to factors outside of stimulation parameters, including the nature of pathology or presence or absence of a structural abnormality (Li and Cook, 2018).
The FDA approval of the Medtronic System for thalamic stimulation has created the opportunity for application of DBS to other thalamic nuclei (Fisher et al., 2010, Shafer, 2018). For example, in other epilepsy conditions such as generalized epilepsy, electrodes are placed in the centromedian nucleus (CM) of the thalamus (Son et al., 2016). We provide a few examples of potential cognition-related questions that can be tested using such recording devices implanted in alternate thalamic targets, in Section 5.0.
4. Outlook and future directions: devices and opportunities for thalamic data collection based on current research findings
4.1. Deep brain stimulation (DBS)
Although an effective clinical intervention, the underlying mechanism to DBS, including ANT-DBS, remains controversial, as studies have yielded seemingly conflicting evidence regarding whether it inhibits or excites local neuronal populations. One review suggests that DBS dissociates incoming and outgoing signals, ultimately disrupting the flow of information in the stimulated site (Chiken and Nambu, 2016). In hippocampal slice model systems (Durand, 1986), high frequency DBS causes negative slow potential shifts and increased extracellular potassium ion concentration, ultimately leading to decreased neuronal excitability.
DBS systems' capability to be turned on and off enables investigators to conduct diverse trials, including randomized and blinded designs, to investigate the unintended effects of stimulation on cognition. Furthermore, modern DBS devices are bidirectional. These devices offer enhanced precision and individualization in treatment approaches by providing both electrical stimulation and recording of brain activity. This capability allows for the exploration of new insights into disease mechanisms and brain networks, specifically in thalamic nuclei (Swann et al., 2018).
Intracranial electrode placement with DBS devices yields recordings with favorably high signal-to-noise ratios and high temporal resolution relative to alternative methods for neural data collection. For example, magnetoencephalography (MEG) localized recordings from small, deep brain structures are infeasible (Singh, 2014). There is a similar issue when working with blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) data (Hall et al., 2016). With regard to temporal resolution of DBS recordings, the high temporal resolution seen in DBS does not depend on the DBS device used itself. Rather, it is a result of the nature of the electrodes and equipment used during DBS. Additionally, DBS provides excellent spatial resolution when electrodes are well-targeted and appropriate reconstructions confirm recording fidelity.
Several open-source toolboxes currently exist that assist with executing experiments using DBS devices. These tools help extract raw data from the device and convert it into useable data for conventional analyses and will perform numerous analyses themselves (Baniasadi et al., 2020; Horn and Kühn, 2015; Lio et al., 2018; Sellers et al., 2021). Fig. 3 depicts a typical ANT-DBS set-up.
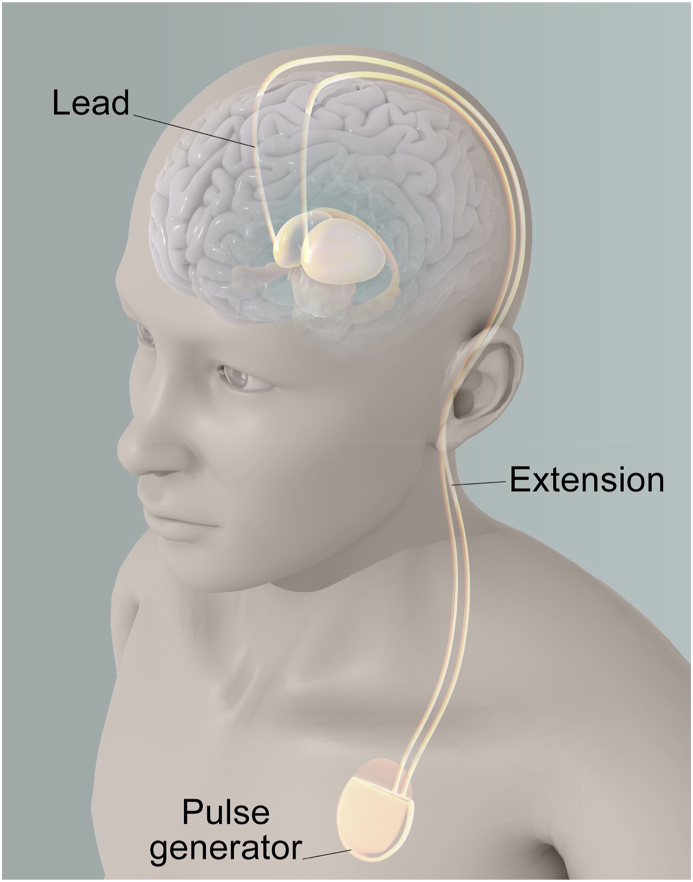
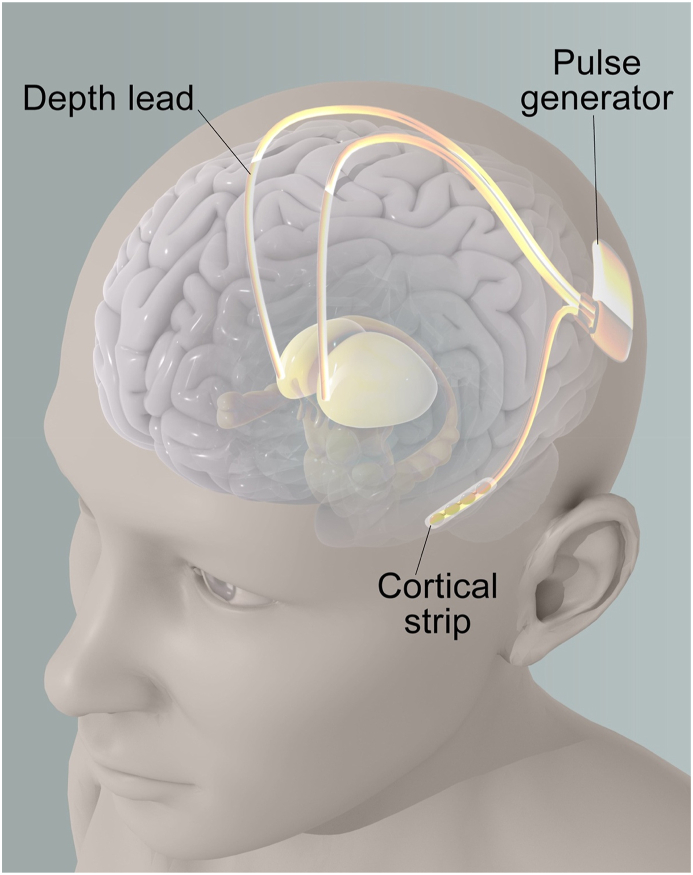
Fig. 3.
Deep Brain Stimulation (DBS) Set Up targeting the ANT
Fig. 3 illustrates a typical deep brain stimulation (DBS) setup, which involves an implanted pulse generator (IPG) and two depth leads that target a specific area of the brain called the anterior thalamic nucleus (ANT). The IPG is typically implanted subcutaneously, often in the chest, and is connected to the depth leads by extension wires that run under the skin. The depth leads are surgically implanted into the brain and are positioned in such a way as to deliver electrical stimulation to the ANT, which has been shown to be effective in treating various neurological disorders.
4.2. DBS and in-Vivo brain sensing
Until recently, data was mostly available through temporary intraoperative recordings in awake patients undergoing implantation surgery (with leads connected to typical amplifiers, not the internal pulse generator). The use of DBS outside of the OR setting for cognitive research has fostered an interest in neural recordings over longer periods of time (Gilron et al., 2021). This in turn creates the opportunity to design experiments that incorporate a broader range of neural phenomena, such as memory consolidation or navigation in real—world situations. These types of experiments represent the “competitive advantage” of chronically implanted systems.
The first “sensing capable” DBS devices were chronically implanted bidirectional devices capable of closed loop neurostimulation. Like prior DBS iterations, this system consists of two leads that are implanted cranially, with a neurostimulator implanted in the chest that can detect neural activity, performing computations and delivering closed or open loop stimulation based on the user programmed parameters. This sensing capability provides clinicians and investigators the ability to track neural activity across a range of diseases and symptom states, with the ability to provide stimulation in response to real-time changes in biomarkers of brain activity (Bouthour et al., 2019; Lo and Widge, 2017; Velisar et al., 2019), as well as the potential to reveal the mechanisms of various cognitive circuits (Gilron et al., 2021).
The utility of neural sensing capabilities is highlighted by biomarker detection. Long-term recordings are an important tool in the identification of new biomarkers for various neurological conditions. In addition to the identification of new biomarkers, long-term recordings can also be used to validate presumed biomarkers that were originally proposed based on short-term, in-hospital recordings. Long-term recordings provide a more comprehensive picture of the patient's neurological condition, allowing for the detection of patterns and trends that may be missed in short-term recordings. This can lead to a deeper understanding of the underlying neurological mechanisms, as well as improved patient care and treatment outcomes. The growing availability and capability of long-term recording devices have greatly expanded the potential for identifying biomarkers and improving our understanding of neurological conditions (Gilron et al., 2021).
Gilron et al. reported on the first human use of the Medtronic RC + S device, with the capability for wireless streaming of field potentials, over an extended period, with and/or without the use of simultaneous neurostimulation, in PD patients. Through this approach, limited side effects were noted, and it was established that multiple recording sites within the cortex and basal ganglia improved the classification of a PD patient's motor state (Gilron et al., 2021; Swann et al., 2018). This analysis demonstrates the utility in obtaining chronic recordings from the ANT during memory tasks. Apart from standard experiments, these devices can record oscillatory activity during more naturalistic memory experiences, with incidental encoding. The incorporation of stimulation routines into these experiments is also feasible once a clear relationship between acute stimulation and memory-related changes are identified.
One newer addition to the sensing-capable DBS market is the Medtronic Percept, which is capable of in-vivo brain sensing (Jimenez-Shahed, 2021). This device can survey LFP activity using its “BrainSense” feature. This is carried out with stimulation OFF, and a graph of the differential in LFP signal between contact pairs can be generated (Jimenez-Shahed, 2021). The difference lies in its pulse generator software that can process and analyze LFPs in real-time allowing them to be stored for later use by the clinician, as well as transmit neural data via Bluetooth telemetry to be synced with behavioral data using gross timestamps. It should be noted that the latter requires specialized equipment to interface with the clinical system, that must be provided by the manufacturer under data use agreements. This in turn requires an estimated 6-month waiting period to begin cognitive experimentation using these devices, which must be kept in mind by potential investigators.
Along with its novel sensing functionality, it features event logging capabilities that allow patients to view the neurophysiological characteristics associated with their symptoms and can be utilized for syncing with experimental paradigms. The native functionality includes responsive stimulation via detection of LFPs. The device is also 3 T MR compatible, which permits the analysis of post-stimulation BOLD signal changes (Jimenez-Shahed, 2021). This may facilitate understanding of brain-wide network changes associated with ANT stimulation. This modern DBS device can thus stream LFPs in near real time using telemetry, analogous to the RNS device, which is discussed in the next section, Section 4.3.
An in-vivo neural sensing experiment in an individual with PD and DBS to the globus pallidus was conducted to test closed-loop stimulation via the Percept PC Device. When compared to open-loop stimulation, closed-loop stimulation showed significant reduction in the LFP beta-band power, an exploratory biomarker in PD, suggesting that the closed-loop functionality was operable (Cummins et al., 2021). Similar experiments are feasible once biomarkers of interest (for example, theta power) are identified for mnemonically relevant behavior in the ANT. To date, one published study used the Percept PC device to analyze the ANT regarding automatisms and corresponding brain activity (Lopes et al., 2022). The protocol involved one participant, coupled with the analysis of their LFPs via DBS with simultaneous video electroencephalography over a 5-day time frame (Lopes et al., 2022). The study conducted time-frequency mapping and event-related desynchronization/synchronization analysis to demonstrate the involvement of the ANT in the execution of automatisms, as evidenced by synchronized activity in the 7–17 Hz map corresponding to hand rotations. These findings provide a foundation for further investigation of the ANT in seizure-related motion and pave the way for integrating in-vivo brain-sensing DBS devices into cognitively focused human experiments. The study represents the first of its kind in this area (Lopes et al., 2022).
One point of clarification is that some surgeons use temporary, externalized leads implanted to the ANT for stimulation optimization prior to conversion to the fully implanted system. In such cases, the ANT electrodes can be connected to conventional recording systems for behavioral experiments analogous to those used for SEEG electrodes.
While the sensing capabilities of these newer devices can permit the recording of behaviorally related activity in the ANT, we note that a classical experiment conducted using DBS devices utilized only stimulation to alter reward circuitry and thereby affect behavior (Frank et al., 2007).
Many common analysis in neural signal processing in humans such as local field potentials (LFP) and cross-frequency phase amplitude coupling (cfPAC), are limited to data obtained from limited-time SEEG recordings (Buzsáki, 2010). However, with DBS systems that can record neural activity, these can be revisited in the thalamus and over longer periods of time. For example, an analysis of recordings from the ANT revealed that cfPAC corresponding to phase synchrony variations is localized to the thalamus in the same hemisphere of the observed seizure activity (Ibrahim et al., 2018). Consequently, these alterations in cfPAC are considerable targets in patient-specific neuromodulation models for individuals with refractory epilepsy. Additionally, thalamic DBS has revealed the occurrence of PAC and amplitude-amplitude coupling (AAC) across various frequencies in the human thalamus, and between oscillations within the cortex and thalamus, identifying a possible explanation for information relay between the regions; PAC has been observed within the ANT as well as between the ANT and frontal cortex (Fitzgerald et al., 2013; Sweeney-Reed et al., 2014, 2017). Furthermore, spiking activity has been recorded in order to localize deep brain structures via microelectrodes, while recording LFP data for subsequent power analysis via a DBS macroelectrode (Lega et al., 2011). These data demonstrate the feasibility of measuring PAC using DBS electrodes, and its utility in informing treatment although this study utilized externalized leads in the intraoperative environment.
The DBS “sensing” feature capable by the above devices has limitations. Namely, gamma frequencies higher than 30 Hz are likely unreliable given the lack of preamplification prior to the connection to extension wires. Sync-pulsing strategies and approaches also vary. One option is to use intermittent programmed stimulation pulses that can also be detected on a conventionally synced clinical recording system such as a surface EEG, for example. Using conventional, clinical-type device control tools, external triggering can only work for initiating a train of pulses. Thus, this necessitates the presence of some other mechanism to capture the stimulation pulses for aligning captured data. This may achieve temporal precision on the order of approximately 20 ms. Such sync pulse limitations may therefore be more useful for comparison among longer temporal epochs rather than events-defined differences in behavior as in traditional, controlled memory paradigms.
4.3. Responsive neurostimulation (RNS)
Another form of neuromodulation, known as responsive neurostimulation (RNS), shown in Fig. 4, has also been approved as adjunctive therapy for drug-resistant epilepsy (Fountas and Smith, 2007). The RNS system consists of a cranially implanted pulse generator device with one to two depth leads placed at the seizure focus. This allows for the continuous monitoring of electrocorticographic activity. When abnormal electrographic activity is sensed, the stimulator delivers electrical pulses within milliseconds to the leads at the seizure focus (Sun and Morrell, 2014). This system differs from DBS in that it is built from the ground up as a closed-loop stimulation device, requiring the acquisition of real time brain signals and online analysis of waveforms to test for and respond to seizure activity. High frequency acquisition and resolution is also native to the system, unlike the DBS device. In a recently initiated clinical trial, the NeuroPace RNS system is being utilized to stimulate structures in the thalamus (Morrell, 2022; Topalovic et al., 2020). The trial utilizes stimulation using both the approach of responsive stimulation patterns typical of the RNS, as well as the long stimulation “ON” times that mimic the DBS approach (Morrell, 2022, Topalovic et al., 2020). Sync pulsing for behavioral experiments requires a custom-built device from the company, usually borrowed via a data use agreement. RNS devices delivering sync pulses are able to timestamp them in the clinical system. The maximum epoch that can be saved on the RNS device is two 240-s time epochs, but the device can also continuously transmit signal telemetrically using an external wand. However, using block design requires a limit of around 4 min in order to facilitate new sets of sync pulses.
Fig. 4.
Responsive Neurostimulation (RNS) Set Up targeting the ANT
Fig. 4 shows a typical setup for Responsive Neurostimulation (RNS), which involves a cranially implanted pulse generator, a cortical strip, and two depth leads targeting the seizure focus, in this case the anterior nucleus of the thalamus (ANT). The pulse generator is placed beneath the scalp and connected to the leads via extension wires. The cortical strip is placed on the surface of the brain and records brain activity, which is used to trigger stimulation from the pulse generator to prevent or reduce seizures. RNS is an FDA-approved treatment for refractory epilepsy and has shown promising results in clinical trials.
Groups that have used this approach for research have devised backpack-like apparatuses that place the wand over the RNS device for continuous streaming (Topalovic et al., 2020). The continuous streaming allows the RNS device to be tested in external, natural environments, offering the ability to synchronize oscillatory recordings with behavioral events (Morrell, 2022, Sun and Morrell, 2014), and creating opportunities to investigate behavioral modalities not available with other systems, such as DBS or Vagal Nerve Stimulators (VNS) (Topalovic et al., 2020).
Published studies on the RNS device and epilepsy are limited to patients with 1–2 seizure foci, with the treatment targeting the zone in which the seizure is first produced (Elder et al., 2019). There is uncertainty about whether the RNS system must directly stimulate the cerebral cortex or if it would be feasible to stimulate non-cortical brain regions. Its usage in targeting the ANT is under consideration, with hopes that its thalamocortical projections to the neocortex can have a neuromodulatory role in seizure treatment (Elder et al., 2019). Presumably, if the RNS of a brain region with various projections to the cortex can modulate activity at distal cortical foci, this may act as an intervention for patients with generalized epilepsy, or epilepsy with multiple foci.
Elder et al. (2019) describes a study of three patients with drug-resistant multifocal epilepsy. These patients underwent RNS implantation, including a unilateral stimulation point to the ANT. Follow-up evaluations reported a decrease in seizure activity in all study patients, with no adverse effect on the patient behavior, mood, or memory. Thus, though the reduction was modest, the study found RNS of the ANT to be a feasible and well tolerated protocol, necessitating further studies to streamline the optimal stimulation conditions. This study demonstrated the ability for RNS electrodes in the ANT to record and enable visualization of thalamic coupling for the seizures of the patients studied. Being able to record from the ANT and target its many networks and pathways on an individualized and stimulus-dependent scale, makes the RNS a highly considerable intervention strategy.
More recently, the RNS System was used in a pilot experiment regarding obesity. Laboratory and real-world neural observations have found that nucleus accumbens (NAc) low frequency oscillatory power is associated with loss of control (LOC) eating, acting as a biomarker. Using this target, a 6-month stimulation phase implicated bilateral NAc-RNS to two subjects with binge eating disorder (BED) and extreme obesity. RNS stimulation resulted in an improvement of LOC eating and a reduction in body weight and BMI (Shivacharan et al., 2022). These results suggest that electrophysiologically targeted RNS can restore inhibitory control in regions such as the NAc. More importantly, it shows the capability of the RNS device to not only identify an oscillatory biomarker, but to target it for therapeutic stimulation. On a larger scale, this remarkable potential may inform interventional approaches for many episodic neurological disorders with a concurrent biomarker.
Possible areas of future investigation by RNS may utilize its ability to record chronically and test multiple times per day. With the ability to test in a natural environment, there is chance to create novel episodic memory paradigms to further reveal the contributions of the ANT to consolidation.
4.4. A unique application of RNS with virtual reality
Using the RNS System, one research group added additional combinations of embedded electronics and software scripts to create a one-of-a-kind system they denoted as Mo-DBRS (Topalovic et al., 2020). This platform is intended primarily to be used with the RNS system, but its elements can be adopted for use with other implanted DBS. Its interface allows for wireless, programmable intracranial electroencephalography to be synchronized with biophysical data. The experimental apparatus includes a backpack to permit sync pulsing while patients are mobile and data streaming to overcome the onboard limits on recordings for the RNS. Researchers were able to use this apparatus in conjunction with virtual reality, to create a testing space that simulates a natural environment (Topalovic et al., 2020). Using this method, RNS electrode placement in the ANT can be used to clarify the role of the ANT in the process of spatial navigation.
The preamplification of brain signal on intracranial electrodes offered by the RNS permits recordings in the gamma frequency range, which in turn facilitates cross frequency coupling analyses. Hence, compared to DBS, RNS devices offer the advantage of recording neural activity at higher frequencies.
4.5. Stereoelectroencephalography (SEEG)
Both DBS and RNS are used as therapeutic devices that offer research opportunities, whereas stereo electroencephalography (SEEG) is a diagnostic tool. While much more invasive than traditional scalp electroencephalography (EEG), SEEG provides a unique mechanism for seizure foci localization and mapping of functional networks with high spatiotemporal resolution (Fig. 5). SEEG leads are implanted to create a four-dimensional map of seizure activity to guide subsequent therapy. Stereotactic procedures involving the thalamus specifically can be traced back to the mid-20th century, when thalamotomy was first reported as a neurological intervention, and the thalamus was described as a zone for epileptic activity (Chaitanya et al., 2020). With the potential advantage of using thalamic stimulation as an intervention strategy, epilepsy centers have started to use precisely target SEEG leads to record from the thalamus. The potential impact of stimulation pulses on interictal activity can be assessed using these recordings (Chaitanya et al., 2020). As such, nascent SEEG recordings from the thalamus will permit the incorporation of existing testing infrastructure that has been established over two decades of human research.
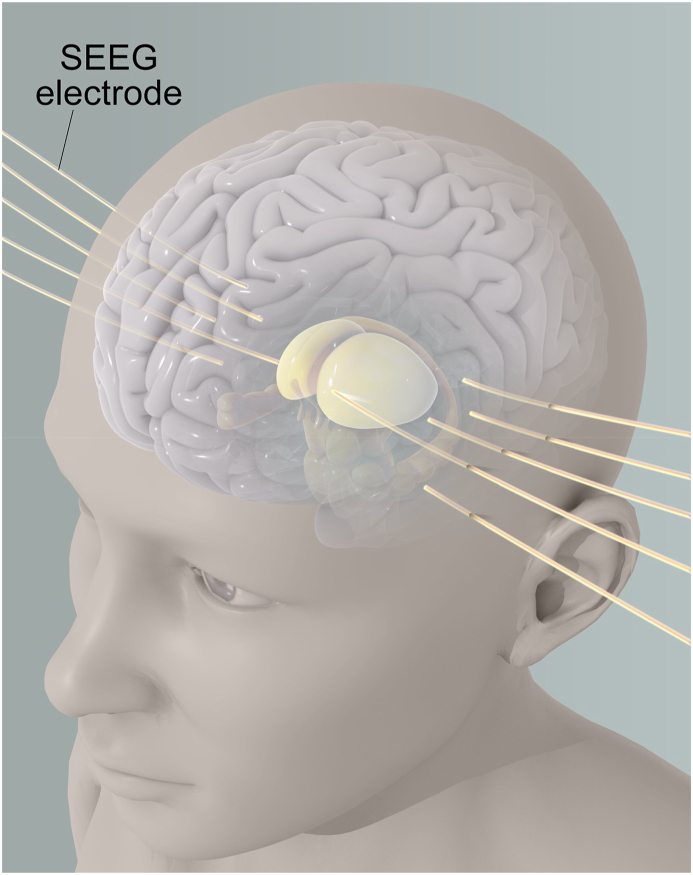
Fig. 5.
Stereo EEG (SEEG) Set Up targeting the ANT
Fig. 5 depicts a setup for stereoelectroencephalography (SEEG), which involves the invasive placement of electrodes to map functional networks in the brain at a high spatial resolution. In this example, one electrode is shown targeting the anterior thalamic nucleus (ANT) specifically. SEEG involves the placement of multiple depth electrodes directly into the brain tissue to record electrical activity from within the brain. SEEG is an effective tool for localizing the epileptic focus and planning surgical interventions for patients with drug-resistant epilepsy. The high spatial resolution of SEEG can also be used to map functional networks involved in other neurological disorders.
After electrodes are inserted during an SEEG procedure, patients are required to stay in the hospital for a few days. During this time, their anti-seizure medications are gradually reduced, and their seizures are recorded. Due to the extended hospital stay, patients often participate in behavioral experiments while undergoing recording from SEEG, with the aim of linking behavior to specific electrophysiological phenomena. Unfortunately, there is a shortage of electrophysiological studies on the thalamus, including the ANT. The number of surgical cases that are sampled for ANT are generally low and vary by center. Therefore, it may be necessary to conduct multi-site cooperative studies to collect data within reasonable time frames.
The accuracy of SEEG electrodes, which are thinner and less rigid than DBS electrodes, make targeting the ANT more difficult. One study recruited considerably homogenous groups of patients with temporal lobe epilepsy (TLE) who were eligible for SEEG. Neurosurgeons modified the trajectory of one of the electrodes planned for clinical sampling to extend to the thalamus and successfully sampled the ANT in 10 out of the 13 patients enrolled. There were few complications as a result, and post-procedural imaging found that none of the patients experienced thalamic hemorrhage or edema (Chaitanya et al., 2020). These findings demonstrate a favorable safety profile of this procedure, creating potential for more routine ANT recordings in SEEG. The SEEG method is particularly valuable because it provides simultaneous access to anatomically distant but functionally related areas (Toth et al., 2020). The use of SEEG to incorporate ANT recordings is an emerging, but potentially powerful platform for data collection to understand ANT physiology during mnemonic processing.
5. Additional thalamic targets considered for DBS
The centromedian (CM), dorsomedial (DM), and pulvinar regions of the thalamus have been tested as potential therapeutic targets for stimulation (Fig. 6). These alternative thalamic regions may see increasing opportunity for stimulation and recording given recent characterization of the cognitive contributions from these areas. Here we aim to provide a concise overview of the understood function of these nuclei, and their involvement in thalamic DBS.
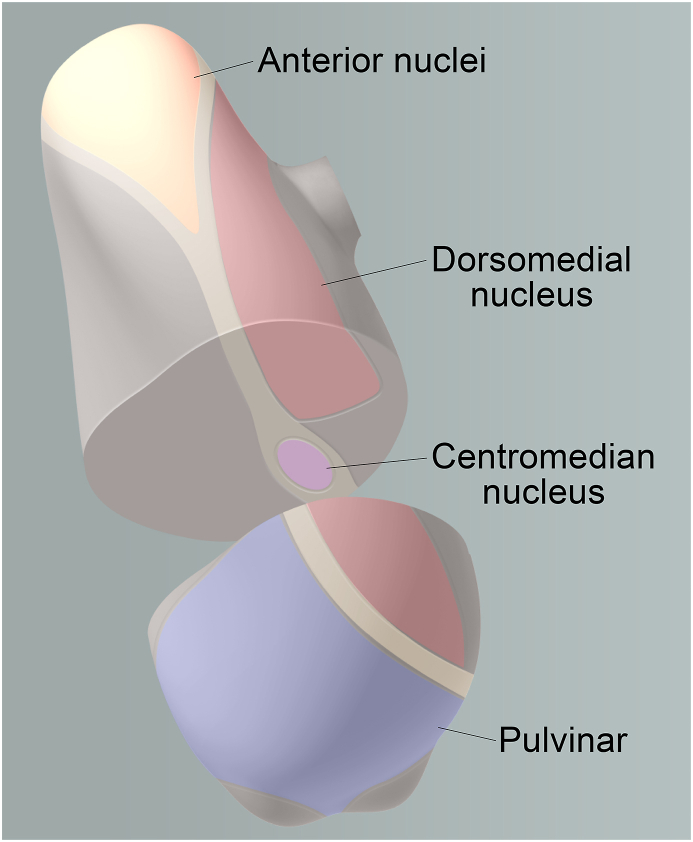
Fig. 6.
Overview of Thalamic Regions and Deep Brain Stimulation Targets
This figure provides an overview of the thalamus and its various regions, with a focus on the anterior thalamic nucleus (ANT), which is the main target of our study. Other deep brain stimulation targets, such as the dorsomedial nucleus, centromedian nucleus, and pulvinar, are also highlighted. The boundaries of each region are demarcated in a sagittal view of the brain, with corresponding coronal sections showing the location of targets, specifically the centromedian nucleus, within the thalamus.
5.1. The centromedian nucleus (CM)
The CM nucleus belongs to the caudal cluster of intralaminar thalamic nuclei and is involved in a widespread network that connects to the ascending reticular system in the brainstem, basal ganglia, direct cortical projections, and other thalamic nuclei through interconnections (Cukiert et al., 2020; Velasco et al., 1989, 1993, 2021). Several studies have shown the efficacy of DBS on the CM for seizure reduction (Son et al., 2016; Velasco et al., 2000). CM stimulation has been observed to improve seizure frequency in patients with Lennox-Gastaut Syndrome generalized drug-resistant epilepsy by over 50 percent (Son et al., 2016), as well as improvement in tonic-clonic seizures in the same patient population (Velasco et al., 2000). Similar results have been seen in patients with multifocal epilepsy (Son et al., 2016).
Physiologically, the CM has a potential functional role in motor planning, pain processing, and sensorimotor coordination, and has recently been explored as a therapeutic neuromodulatory target for Parkinson's Disease, neuropathic pain, Tourette Syndrome, as well as for restoring consciousness (Vetkas et al., 2022; Vetkas et al., 2022). While not as efficacious as conventional DBS to the STN, patients with tremor-dominant PD that is resistant to STN stimulation can find significant relief with DBS targeting the CM and parafascicular thalamic nucleus (Pf) (Stefani et al., 2009). DBS to the CM and Pf has also shown efficacy in reduction of motor and vocal tics (Martinez-Ramirez et al., 2018).
The CM being proposed as a plausible target in DRE, makes for an interesting comparison to the ANT. In fact, a retrospective case series reported that patients who received simultaneous CM + ANT DBS did not show any significant difference in the median seizure frequency reduction and responder rate compared to those who received CM-DBS alone (Alcala-Zermeno et al., 2021). These findings indicate a need for additional controlled studies to evaluate the effectiveness of DBS for generalized, combined generalized and focal, and poorly localized or posterior onset focal epilepsies using both CM and CM + ANT stimulation.
Additionally, the CM has been suggested to be an effective DBS target in patients with drug resistant genetic generalized epilepsy (GGE), demonstrated in a case study on a 27-year-old cognitively normal woman with GGE (Agashe et al., 2022). Notably, only two studies of CM-DBS have been carried out in cognitively normal individuals with epilepsy, necessitating a need for further study of the CM utilizing DBS (Agashe et al., 2022; Cukiert et al., 2020; Valentín et al., 2013). Similar to DBS of the ANT, CM-DBS can provide valuable insights into the function of the CM, which can contribute to a better understanding of the broader thalamic network and its role in various neurological conditions.
5.2. The dorsomedial nucleus (DM)
The DM is known for its complex and extensive interconnections with other brain regions, namely the prefrontal cortex (PFC) and limbic structures (Georgescu et al., 2020; Ouhaz et al., 2018), allowing it to impact various aspects of cognition and behavior. A well-studied consequence of its dysfunction is demonstrated in models of schizophrenia.
In a schizophrenia model in mice, inhibition of the DM impaired working memory tasks (Parnaudeau et al., 2013). Moreover, DM-DBS was demonstrated as beneficial for schizophrenia in an animal model (Klein et al., 2013). Similarly, DM dysfunction has been linked with cognitive impairment in human schizophrenic patients (Callicott et al., 2003; Hazlett et al., 2000; Lewis, 2000; Schröder et al., 1996).
Given the potential association between hippocampal circuitry and schizophrenia in animal models of the disease (Floresco and Grace, 2003; Klein et al., 2013; Parnaudeau et al., 2013), DBS electrodes in the DM may create opportunities for investigating hippocampal responses to DM neuromodulation during tasks such as mnemonic similarity. This paradigm highlights specific memory derangement observed in patients with schizophrenia (Alelú-Paz and Giménez-Amaya, 2008). Disruptive stimulation applied to the MD would be expected to elicit downstream changes in the hippocampus that could be correlated with behavioral modulations.
The DM nucleus likely plays some complex role in regulating prefrontal cortex-hippocampal activity as well. A study in mice directly stimulated the DM and ventral tegmental area while recording from the hippocampus and prefrontal cortex. The authors found varying effects, based on the paradigm and parameters of stimulation. Based on their data, the study reported that an extended elevation in DM activity could enhance working memory functions by increasing the responsiveness of PFC neurons to information from the hippocampus, even if there is a delay (Floresco and Grace, 2003). The authors interpret their findings to mean that the function of the DM is to modulate the transmission of information between the frontal lobes and hippocampus. Consequently, disruptive stimulation applied to the DM may be expected to alter behavior in paradigms that require top-down control of attention or related processes that alter hippocampal inputs.
5.3. The pulvinar
The pulvinar, located in the posterior thalamus and being the largest thalamic nucleus and possessing extensive connectivity with other brain regions (Benazzouz and Hallett, 2000), plays a crucial role in processing different types of sensory information. Specifically, the lateral pulvinar is mainly associated with the visual system, whereas the dorsomedial pulvinar is linked to parietal regions. Moreover, the medial pulvinar is connected to several brain regions, such as the amygdala, hippocampus, temporal neocortex, cingulate, and orbitofrontal cortex. It has been observed that pulvinar units become active in response to movements, auditory stimuli, visual stimuli, and even list stimuli, such as letters and numbers (Magariños-Ascone et al., 1988, Schneider et al., 2020), highlighting the importance of pulvinar activity in the processing of sensory information.
In response to movements, auditory stimuli, or visual stimuli, pulvinar units activate (Magariños-Ascone et al., 1988). Moreover, recent research has also suggested that the pulvinar is involved in processing list stimuli, such as letters and numbers, as pulvinar activity has been observed in response to these stimuli(Schneider et al., 2020). This indicates that the pulvinar's role in sensory processing extends beyond traditional visual and auditory stimuli. Additionally, current literature proposes the main cognitive function of the pulvinar as being selective attention and visual attention filtering (Fama and Sullivan, 2015), with a role in consciousness. Thus, with its extensive connections to other brain regions, the pulvinar stands out as a key component of the thalamus, and a better understanding of its function could provide insights into the underlying mechanisms of various neurological conditions.
The pulvinar was not investigated as a DBS target for DRE until recently (Filipescu et al., 2019). Based on direct recordings, the pulvinar has been demonstrated to regulate cortical synchrony in relation to attentional demand, indicating a role in the transfer of information throughout the visual cortex (Saalmann et al., 2012). The pulvinar contributes to thalamocortical oscillations that modulate cortical synchronization, which are proposed targets for DBS to reduce seizure-related synchrony (Filipescu et al., 2019); based on direct pulvinar recordings, regulation of cortical synchrony by the pulvinar occurs according to attentional demand. Using SEEG, one study looked at the effects of stimulation on this region of the thalamus of patients who underwent temporal-lobe seizure inducement (Filipescu et al., 2019). Their results showed the tonic phase of ictal discharge was affected by stimulation, and the impact on alterations in awareness of seizure events was reduced.
It had been suggested that the pulvinar may be a suitable target for treating refractory seizures that originate from the posterior part of the brain. Using SEEG, researchers deduced that the functional connectivity of the pulvinar nuclei in the human brain aligns with the majority of findings from anatomical studies in primates (Rosenberg et al., 2008). However, there were a few notable discrepancies, such as a robust functional connection between the amygdala-hippocampal complex and pulvinar nuclei, as well as an unforeseen asymmetry in certain reciprocal pathways examined. These findings provide greater insight into the functional significance of the pulvinar nuclei and its involvement in temporal lobe epilepsy.
Currently, there is an ongoing clinical trial (Arnaud, 2021), targeting the medial pulvinar for DRE based on retrospective studies that have shown the involvement of the medial pulvinar during focal seizures and in the termination of seizures and loss of consciousness. Recent feasibility and safety studies have also provided promising results for medial pulvinar stimulation (Arnaud, 2021).
Per current proposals on the pulvinar's involvement in selective attention and visual attention filtering (Fama and Sullivan, 2015), the potential association between the pulvinar and disorders of consciousness requires further investigation. Possibilities for further examination include observing the impact of pulvinar stimulation on hypersynchrony between the thalamus and frontal and parietal cortexes, which is necessary for processing of consciousness. Recordings during stages of sleep versus the induction of anesthesia should also be feasible, building upon the uniquity of such clinical situations.
6. Conclusion
The ANT is thought to be involved in complex cognitive functions, such as episodic memory (Sweeney-Reed et al., 2021), and spatial navigation (Clark and Taube, 2012), with a diverse set of thalamocortical projections from its subnuclei allowing it to relay and receive an assortment of sensory information. While much of its function remains under investigation, its utility as a DBS target for DRE appears promising.
Recent developments in neuromodulation devices such as DBS, RNS, and SEEG have the potential to provide new and exciting insights into human cognition. This paper discusses how these devices can further our understanding of the ANT circuit in humans, potentially leading to safer and more effective treatments for related diseases. Additionally, exploring the functional circuitry of other thalamic nuclei such as the CM, DM, and pulvinar can also provide valuable information that can be applied to research and clinical settings. The optimal use of these devices necessitates further study but creates opportunities for novel methods of discovery in neuroscience.
Valuable future directions of research include evaluation of the usage of RNS bilaterally in the ANT to measure where seizure onset begins, as it is inconclusive whether seizure onset occurs in the ANT itself. In addition, exploring the ANT through the use of closed-loop DBS experimentation has the potential to yield valuable insights into disease-state biomarkers that are specific to this region, which can then be leveraged as treatment targets. This approach also has the potential to shed light on the broader role of the ANT within larger neural networks, particularly in the context of patients undergoing SEEG investigations.
CRediT authorship contribution statement
Pooja Venkatesh: Conceptualization, Investigation, Data curation, Writing – original draft, Visualization. Cody Wolfe: Conceptualization, Writing – review & editing. Bradley Lega: Conceptualization, Investigation, Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Consultant for NIA Therapeutics - B.L.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No data was used for the research described in the article.
References
- Agashe S., Burkholder D., Starnes K., Van Gompel J.J., Lundstrom B.N., Worrell G.A., Gregg N.M. Centromedian nucleus of the thalamus deep brain stimulation for genetic generalized epilepsy: case report and review of literature. Front. Hum. Neurosci. 2022;16 doi: 10.3389/fnhum.2022.858413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J.P. EPS Mid-Career Award 2006. Understanding anterograde amnesia: disconnections and hidden lesions. Q. J. Exp. Psychol. 2008;61(10):1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. Rev. 2012;36(7):1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., Brown M.W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22(3):425–444. https://www.ncbi.nlm.nih.gov/pubmed/11301518 ; discussion 444-489. [PubMed] [Google Scholar]
- Aggleton J.P., O'Mara S.M. The anterior thalamic nuclei: core components of a tripartite episodic memory system. Nat. Rev. Neurosci. 2022;23(8):505–516. doi: 10.1038/s41583-022-00591-8. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., O'Mara S.M., Vann S.D., Wright N.F., Tsanov M., Erichsen J.T. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur. J. Neurosci. 2010;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala-Zermeno J.L., Gregg N.M., Wirrell E.C., Stead M., Worrell G.A., Van Gompel J.J., Lundstrom B.N. Centromedian thalamic nucleus with or without anterior thalamic nucleus deep brain stimulation for epilepsy in children and adults: a retrospective case series. Seizure. 2021;84:101–107. doi: 10.1016/j.seizure.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alelú-Paz R., Giménez-Amaya J.M. The mediodorsal thalamic nucleus and schizophrenia. J. Psychiatr. Neurosci. 2008;33(6):489–498. [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Cowan W.M. Subcortical afferents to the hippocampal formation in the monkey. J. Comp. Neurol. 1980;189(4):573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Anderson C.J., Anderson D.N., Pulst S.M., Butson C.R., Dorval A.D. Neural selectivity, efficiency, and dose equivalence in deep brain stimulation through pulse width tuning and segmented electrodes. Brain Stimul. 2020;13(4):1040–1050. doi: 10.1016/j.brs.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J.-O. ClinicalTrials.gov National Library of Medicine. U.S.; 2021. Pulvinar stimulation in epilepsy: a pilot study. [Google Scholar]
- Balak N., Balkuv E., Karadag A., Basaran R., Biceroglu H., Erkan B., Tanriover N. Mammillothalamic and mammillotegmental tracts as new targets for dementia and epilepsy treatment. World Neurosurgery. 2018;110:133–144. doi: 10.1016/j.wneu.2017.10.168. [DOI] [PubMed] [Google Scholar]
- Baniasadi M., Proverbio D., Gonçalves J., Hertel F., Husch A. FastField: an open-source toolbox for efficient approximation of deep brain stimulation electric fields. Neuroimage. 2020;223 doi: 10.1016/j.neuroimage.2020.117330. [DOI] [PubMed] [Google Scholar]
- Barbe M.T., Reker P., Hamacher S., Franklin J., Kraus D., Dembek T.A., Becker J., Steffen J.K., Allert N., Wirths J., Dafsari H.S., Voges J., Fink G.R., Visser-Vandewalle V., Timmermann L. DBS of the PSA and the VIM in essential tremor. A randomized, double-blind, crossover trial. 2018;91(6):e543–e550. doi: 10.1212/wnl.0000000000005956. [DOI] [PubMed] [Google Scholar]
- Belasen A., Rizvi K., Gee L.E., Yeung P., Prusik J., Ramirez-Zamora A., Hanspal E., Paiva P., Durphy J., Argoff C.E., Pilitsis J.G. Effect of low-frequency deep brain stimulation on sensory thresholds in Parkinson's disease. Journal of Neurosurgery JNS. 2017;126(2):397–403. doi: 10.3171/2016.2.Jns152231. [DOI] [PubMed] [Google Scholar]
- Benazzouz A., Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55(12 Suppl. 6):S13–S16. [PubMed] [Google Scholar]
- Bouthour W., Megevand P., Donoghue J., Luscher C., Birbaumer N., Krack P. Author Correction: biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019;15(6):363. doi: 10.1038/s41582-019-0189-x. [DOI] [PubMed] [Google Scholar]
- Bubb E.J., Aggleton J.P., O'Mara S.M., Nelson A.J. Chemogenetics reveal an anterior cingulate–thalamic pathway for attending to task-relevant information. Cerebr. Cortex. 2021;31(4):2169–2186. doi: 10.1093/cercor/bhaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68(3):362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Tingley D. Space and time: the hippocampus as a sequence generator. Trends Cognit. Sci. 2018;22(10):853–869. doi: 10.1016/j.tics.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott J.H., Mattay V.S., Verchinski B.A., Marenco S., Egan M.F., Weinberger D.R. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am. J. Psychiatr. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Lombardi M.G., Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49(5):777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Chaitanya G., Romeo A.K., Ilyas A., Irannejad A., Toth E., Elsayed G., Bentley J.N., Riley K.O., Pati S. Robot-assisted stereoelectroencephalography exploration of the limbic thalamus in human focal epilepsy: implantation technique and complications in the first 24 patients. Neurosurg. Focus. 2020;48(4):E2. doi: 10.3171/2020.1.FOCUS19887. [DOI] [PubMed] [Google Scholar]
- Chiken S., Nambu A. Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist. 2016;22(3):313–322. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child N.D., Benarroch E.E. Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology. 2013;81(21):1869–1876. doi: 10.1212/01.wnl.0000436078.95856.56. [DOI] [PubMed] [Google Scholar]
- Clark B.J., Taube J.S. Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Front. Neural Circ. 2012;6:7. doi: 10.3389/fncir.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper I., Upton A., Amin I., Garnett S., Brown G., Springman M. Evoked metabolic responses in the limbic-striate system produced by stimulation of anterior thalamic nucleus in man. Int. J. Neurol. 1984;18:179–187. [PubMed] [Google Scholar]
- Cukiert A., Cukiert C.M., Burattini J.A., Mariani P.P. Seizure outcome during bilateral, continuous, thalamic centromedian nuclei deep brain stimulation in patients with generalized epilepsy: a prospective, open-label study. Seizure. 2020;81:304–309. doi: 10.1016/j.seizure.2020.08.028. [DOI] [PubMed] [Google Scholar]
- Cummins D.D., Kochanski R.B., Gilron R., Swann N.C., Little S., Hammer L.H., Starr P.A. Chronic sensing of subthalamic local field potentials: comparison of first and second generation implantable bidirectional systems within a single subject. Front. Neurosci. 2021;987 doi: 10.3389/fnins.2021.725797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky J.O., Lozano A.M. Mechanisms of deep brain stimulation. Mov. Disord. 2002;17(S3):S63–S68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- Duffley G., Anderson D.N., Vorwerk J., Dorval A.D., Butson C.R. Evaluation of methodologies for computing the deep brain stimulation volume of tissue activated. J. Neural. Eng. 2019;16(6) doi: 10.1088/1741-2552/ab3c95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D. Electrical stimulation can inhibit synchronized neuronal activity. Brain Res. 1986;382(1):139–144. doi: 10.1016/0006-8993(86)90121-6. [DOI] [PubMed] [Google Scholar]
- Edelstyn N., Ellis S., Jenkinson P., Sawyer A. Contribution of the left dorsomedial thalamus to recognition memory: a neuropsychological case study. Neurocase. 2002;8(6):442–452. doi: 10.1076/neur.8.5.442.16180. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat. Rev. Neurosci. 2014;15(11):732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. On the integration of space, time, and memory. Neuron. 2017;95(5):1007–1018. doi: 10.1016/j.neuron.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder C., Friedman D., Devinsky O., Doyle W., Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open. 2019;4(1):187–192. doi: 10.1002/epi4.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsanadidy E., Mosa I.M., Hou B., Schmid T., El-Kady M.F., Khan R.S., Haeberlin A., Tzingounis A.V., Rusling J.F. Self-sustainable intermittent deep brain stimulator. Cell Reports Physical Science. 2022;3(10) [Google Scholar]
- Fama R., Sullivan E.V. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci. Biobehav. Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Daniele A., Albanese A. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol. 2012;11(5):429–442. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- Filipescu C., Lagarde S., Lambert I., Pizzo F., Trébuchon A., McGonigal A., Scavarda D., Carron R., Bartolomei F. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia. 2019;60(4):e25–e30. doi: 10.1111/epi.14677. [DOI] [PubMed] [Google Scholar]
- Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R., Oommen K., Osorio I., Nazzaro J., Labar D., Kaplitt M., Sperling M., Sandok E., Neal J., Handforth A., Stern J., DeSalles A., Chung S., Shetter A.…Group S.S. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T.H., Valentin A., Selway R., Richardson M.P. Cross-frequency coupling within and between the human thalamus and neocortex. Front. Hum. Neurosci. 2013;7:84. doi: 10.3389/fnhum.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S.B., Grace A.A. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J. Neurosci. 2003;23(9):3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountas K.N., Smith J.R. A novel closed-loop stimulation system in the control of focal, medically refractory epilepsy. Acta Neurochir. Suppl. 2007;97(2):357–362. doi: 10.1007/978-3-211-33081-4_41. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Samanta J., Moustafa A.A., Sherman S.J. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Freund B.E., Greco E., Okromelidze L., Mendez J., Tatum W.O., Grewal S.S., Middlebrooks E.H. Clinical outcome of imaging-based programming for anterior thalamic nucleus deep brain stimulation. J. Neurosurg. 2022;1(aop):1–8. doi: 10.3171/2022.7.JNS221116. [DOI] [PubMed] [Google Scholar]
- Frost B.E., Martin S.K., Cafalchio M., Islam M.N., Aggleton J.P., O'Mara S.M. Anterior thalamic inputs are required for subiculum spatial coding, with associated consequences for hippocampal spatial memory. J. Neurosci. 2021;41(30):6511–6525. doi: 10.1523/JNEUROSCI.2868-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu I.A., Popa D., Zagrean L. The anatomical and functional heterogeneity of the mediodorsal thalamus. Brain Sci. 2020;10(9):624. doi: 10.3390/brainsci10090624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev D., Mencinger M., Rajnar R., Mušič P., Benedičič M., Flisar D., Bošnjak R., Mehrkens J., Pirtošek Z., Boetzel K., Trošt M. Long-term effect of bilateral STN-DBS on non-motor symptoms in Parkinson's disease: a four-year observational, prospective study. Park. Relat. Disord. 2021;89:13–16. doi: 10.1016/j.parkreldis.2021.06.017. [DOI] [PubMed] [Google Scholar]
- Gibson B., et al. The head-direction signal is critical for navigation requiring a cognitive map but not for learning a spatial habit. Curr. Biol. 2013;23(16):1536–1540. doi: 10.1016/j.cub.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron R.E., Little S., Perrone R., Wilt R., De Hemptinne C., Yaroshinsky M.S., Racine C.A., Wang S.S., Ostrem J.L., Larson P.S., Wang D.D., Galifianakis N.B., Bledsoe I.O., San Luciano M., Dawes H.E., Worrell G.A., Kremen V., Borton D.A., Denison T., Starr P.A. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson's disease. Nat. Biotechnol. 2021;39(9):1078–1085. doi: 10.1038/s41587-021-00897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman P.H., Mortimer J.T. The effect of stimulus parameters on the recruitment characteristics of direct Nerve stimulation. IEEE Transactions on Biomedical Engineering, BME- 1983;30(7):407–414. doi: 10.1109/tbme.1983.325041. [DOI] [PubMed] [Google Scholar]
- Grossberg S., Pilly P.K. Coordinated learning of grid cell and place cell spatial and temporal properties: multiple scales, attention and oscillations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1635) doi: 10.1098/rstb.2012.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Howarth C., Kurth-Nelson Z., Mishra A. vol. 371. The Royal Society; 2016. (Interpreting BOLD: towards a Dialogue between Cognitive and Cellular Neuroscience). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A., Halliday G., Caine D., Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Hazlett E.A., Buchsbaum M.S., Jeu L.A., Nenadic I., Fleischman M.B., Shihabuddin L., Haznedar M.M., Harvey P.D. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr. Res. 2000;43(1):33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- Hicks R.R., Huerta M.F. Differential thalamic connectivity of rostral and caudal parts of cortical area Fr2 in rats. Brain Res. 1991;568(1–2):325–329. doi: 10.1016/0006-8993(91)91420-6. [DOI] [PubMed] [Google Scholar]
- Horn A., Kühn A.A. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Ibrahim G.M., Wong S., Morgan B.R., Lipsman N., Fallah A., Weil A.G., Krishna V., Wennberg R.A., Lozano A.A. Phase-amplitude coupling within the anterior thalamic nuclei during seizures. J. Neurophysiol. 2018;119(4):1497–1505. doi: 10.1152/jn.00832.2017. [DOI] [PubMed] [Google Scholar]
- Ilyas A., Snyder K.M., Thomas T.M., Tandon N. Optimal targeting of the anterior nucleus of the thalamus for epilepsy: a meta-analysis. J. Neurosurg. 2022;1(aop):1–9. doi: 10.3171/2022.2.JNS212550. [DOI] [PubMed] [Google Scholar]
- Jankowski M.M., Ronnqvist K.C., Tsanov M., Vann S.D., Wright N.F., Erichsen J.T., Aggleton J.P., O'mara S.M. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front. Syst. Neurosci. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Shahed J. Device profile of the percept PC deep brain stimulation system for the treatment of Parkinson's disease and related disorders. Expet Rev. Med. Dev. 2021;18(4):319–332. doi: 10.1080/17434440.2021.1909471. [DOI] [PubMed] [Google Scholar]
- Kerrigan J.F., Litt B., Fisher R.S., Cranstoun S., French J.A., Blum D.E., Dichter M., Shetter A., Baltuch G., Jaggi J., Krone S., Brodie M., Rise M., Graves N. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45(4):346–354. doi: 10.1111/j.0013-9580.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- Klein J., Hadar R., Götz T., Männer A., Eberhardt C., Baldassarri J., Schmidt T.T., Kupsch A., Heinz A., Morgenstern R. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimul. 2013;6(4):490–499. doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Koeppen J.A., Nahravani F., Kramer M., Voges B., House P.M., Gulberti A., Moll C.K.E., Westphal M., Hamel W. Electrical stimulation of the anterior thalamus for epilepsy: clinical outcome and analysis of efficient target. Neuromodulation: Technology at the Neural Interface. 2019;22(4):465–471. doi: 10.1111/ner.12865. [DOI] [PubMed] [Google Scholar]
- Kopelman M., Guinan E., Lewis P. Delusional memory, confabulation, and frontal lobe dysfunction: a case study in de clérambault's syndrome. Neurocase. 1995;1(1):71–77. [Google Scholar]
- Lega B.C., Halpern C.H., Jaggi J.L., Baltuch G.H. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiol. Dis. 2010;38(3):354–360. doi: 10.1016/j.nbd.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Lega B.C., Kahana M.J., Jaggi J., Baltuch G.H., Zaghloul K. Neuronal and oscillatory activity during reward processing in the human ventral striatum. Neuroreport. 2011;22(16):795–800. doi: 10.1097/wnr.0b013e32834b2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki K., Möttönen T., Järventausta K., Katisko J., Tähtinen T., Haapasalo J., Niskakangas T., Kiekara T., Öhman J., Peltola J. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016;9(2):268–275. doi: 10.1016/j.brs.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Lewis D.A. Is there a neuropathology of schizophrenia? Recent findings converge on altered thalamic-prefrontal cortical connectivity. Neuroscientist. 2000;6(3):208–218. [Google Scholar]
- Li M.C.H., Cook M.J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59(2):273–290. doi: 10.1111/epi.13964. [DOI] [PubMed] [Google Scholar]
- Lio G., Thobois S., Ballanger B., Lau B., Boulinguez P. Removing deep brain stimulation artifacts from the electroencephalogram: issues, recommendations and an open-source toolbox. Clin. Neurophysiol. 2018;129(10):2170–2185. doi: 10.1016/j.clinph.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Liu J., Yu T., Wu J., Pan Y., Tan Z., Liu R., Wang X., Ren L., Wang L. Anterior thalamic stimulation improves working memory precision judgments. Brain Stimul. 2021;14(5):1073–1080. doi: 10.1016/j.brs.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Lo M.-C., Widge A.S. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Int. Rev. Psychiatr. 2017;29(2):191–204. doi: 10.1080/09540261.2017.1282438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes E.M., Sampaio A.R., Campos A., Santos A., Rego R., Cunha J.P.S. 2022. Involvement of the Anterior Nucleus of the Thalamus during Focal Automatisms in Epileptic Seizures: A First Evidence Study. 2022. [DOI] [PubMed] [Google Scholar]
- Magariños-Ascone C., Buño W., Jr., García-Austt E. Monkey pulvinar units related to motor activity and sensory response. Brain Res. 1988;445(1):30–38. doi: 10.1016/0006-8993(88)91070-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Ramirez D., Jimenez-Shahed J., Leckman J.F., Porta M., Servello D., Meng F.-G., Kuhn J., Huys D., Baldermann J.C., Foltynie T., Hariz M.I., Joyce E.M., Zrinzo L., Kefalopoulou Z., Silburn P., Coyne T., Mogilner A.Y., Pourfar M.H., Khandhar S.M.…Okun M.S. Efficacy and safety of deep brain stimulation in tourette syndrome. JAMA Neurol. 2018;75(3):353. doi: 10.1001/jamaneurol.2017.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks E.H., Lin C., Okromelidze L., Lu C.Q., Tatum W.O., Wharen R.E., Jr., Grewal S.S. Functional activation patterns of deep brain stimulation of the anterior nucleus of the thalamus. World Neurosurg. 2020;136:357–363.e352. doi: 10.1016/j.wneu.2020.01.108. [DOI] [PubMed] [Google Scholar]
- Morrell M. ClinicalTrials.gov National Library of Medicine. U.S.; 2022. RNS system NAUTILUS study. [Google Scholar]
- Möttönen T., Katisko J., Haapasalo J., Tähtinen T., Kiekara T., Kähärä V., Peltola J., Öhman J., Lehtimäki K. Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: delineation using 3 T MRI and intraoperative microelectrode recording. Neuroimage: Clinica. 2015;7:823–829. doi: 10.1016/j.nicl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir G.M., Brown J.E., Carey J.P., Hirvonen T.P., Della Santina C.C., Minor L.B., Taube J.S. Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J. Neurosci. 2009;29(46):14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R.U., et al. Head direction cells: properties and functional significance. Curr. Opinion. Neurobiol. 1996;6(2):196–206. doi: 10.1016/s0959-4388(96)80073-0. [DOI] [PubMed] [Google Scholar]
- Negida A., Elminawy M., El Ashal G., Essam A., Eysa A., Abd Elalem Aziz M. Subthalamic and pallidal deep brain stimulation for Parkinson's disease. Cureus. 2018;10(2):e2232. doi: 10.7759/cureus.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A.J. The anterior thalamic nuclei and cognition: a role beyond space? Neurosci. Biobehav. Rev. 2021;126:1–11. doi: 10.1016/j.neubiorev.2021.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J., Burgess N., Donnett J.G., Jeffery K.J., Maguire E.A. Place cells, navigational accuracy, and the human hippocampus. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 1998;353(1373):1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J., Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oh Y.-S., Kim H.J., Lee K.J., Kim Y.I., Lim S.-C., Shon Y.-M. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. 2012;21(3):183–187. doi: 10.1016/j.seizure.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Ouhaz Z., Fleming H., Mitchell A.S. Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and mediodorsal thalamus. Front. Neurosci. 2018;12:33. doi: 10.3389/fnins.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S., O’neill P.-K., Bolkan S.S., Ward R.D., Abbas A.I., Roth B.L., Balsam P.D., Gordon J.A., Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77(6):1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B.A.L., Mitchell A.S. Considering the evidence for anterior and laterodorsal thalamic nuclei as higher order relays to cortex. Front. Mol. Neurosci. 2019;12:167. doi: 10.3389/fnmol.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.C., Reddy V., Mayes D.A. 2019. Neuroanatomy, Mammillary Bodies. [PubMed] [Google Scholar]
- Peyrache A., Duszkiewicz A.J., Viejo G., Angeles-Duran S. Thalamocortical processing of the head-direction sense. Prog. Neurobiol. 2019;183 doi: 10.1016/j.pneurobio.2019.101693. [DOI] [PubMed] [Google Scholar]
- Ramasubbu R., Lang S., Kiss Z.H. Dosing of electrical parameters in deep brain stimulation (DBS) for intractable depression: a review of clinical studies. Front. Psychiatr. 2018;9:302. doi: 10.3389/fpsyt.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzone M., Lanotte M., Bergamasco B., Tavella A., Torre E., Faccani G., Melcarne A., Lopiano L. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: effects of variation in stimulation parameters. J. Neurol. Neurosurg. Psychiatr. 2001;71(2):215–219. doi: 10.1136/jnnp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D.S., Mauguière F., Catenoix H., Faillenot I., Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cerebr. Cortex. 2008;19(6):1462–1473. doi: 10.1093/cercor/bhn185. [DOI] [PubMed] [Google Scholar]
- Roy D.S., Zhang Y., Aida T., Shen C., Skaggs K.M., Hou Y., Fleishman M., Mosto O., Weninger A., Feng G. Anterior thalamic circuits crucial for working memory. Proc. Natl. Acad. Sci. USA. 2022;119(20) doi: 10.1073/pnas.2118712119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y.B., Pinsk M.A., Wang L., Li X., Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M., Labar D., Sperling M.R., Sharan A., Sandok E., Handforth A., Stern J.M., Chung S., Henderson J.M., French J., Baltuch G., Rosenfeld W.E., Garcia P., Barbaro N.M.…Fisher R. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017–1025. doi: 10.1212/wnl.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Bewernick B.H., Kayser S., Mädler B., Coenen V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatr. 2013;73(12):1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Schneider L., Dominguez-Vargas A.U., Gibson L., Kagan I., Wilke M. Eye position signals in the dorsal pulvinar during fixation and goal-directed saccades. J. Neurophysiol. 2020;123(1):367–391. doi: 10.1152/jn.00432.2019. [DOI] [PubMed] [Google Scholar]
- Schröder J., Buchsbaum M.S., Siegel B.V., Geider F.J., Lohr J., Tang C., Wu J., Potkin S.G. Cerebral metabolic activity correlates of subsyndromes in chronic schizophrenia. Schizophr. Res. 1996;19(1):41–53. doi: 10.1016/0920-9964(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Segobin S., Laniepce A., Ritz L., Lannuzel C., Boudehent C., Cabe N., Urso L., Vabret F., Eustache F., Beaunieux H. Dissociating thalamic alterations in alcohol use disorder defines specificity of Korsakoff's syndrome. Brain. 2019;142(5):1458–1470. doi: 10.1093/brain/awz056. [DOI] [PubMed] [Google Scholar]
- Sellers K.K., Gilron R.e., Anso J., Louie K.H., Shirvalkar P.R., Chang E.F., Little S.J., Starr P.A. Analysis-rcs-data: open-source toolbox for the ingestion, time-alignment, and visualization of sense and stimulation data from the Medtronic Summit RC+ S system. Front. Hum. Neurosci. 2021;15:398. doi: 10.3389/fnhum.2021.714256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer P.O. Epilepsy Foundation; 2018. FDA Approval: Medtronic Deep Brain Stimulation for Medically Refractory Epilepsy.https://www.epilepsy.com/stories/fda-approval-medtronic-deep-brain-stimulation-medically-refractory-epilepsy Tuesday, May 01, 2018. [Google Scholar]
- Sharp P.E., Blair H.T., Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci. 2001;24(5):289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Sheth S.A., Bijanki K.R., Metzger B., Allawala A., Pirtle V., Adkinson J.A., Myers J., Mathura R.K., Oswalt D., Tsolaki E., Xiao J., Noecker A., Strutt A.M., Cohn J.F., McIntyre C.C., Mathew S.J., Borton D., Goodman W., Pouratian N. Deep brain stimulation for depression informed by intracranial recordings. Biol. Psychiatr. 2022;92(3):246–251. doi: 10.1016/j.biopsych.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivacharan R.S., Rolle C.E., Barbosa D.A.N., Cunningham T.N., Feng A., Johnson N.D., Safer D.L., Bohon C., Keller C., Buch V.P., Parker J.J., Azagury D.E., Tass P.A., Bhati M.T., Malenka R.C., Lock J.D., Halpern C.H. Pilot study of responsive nucleus accumbens deep brain stimulation for loss-of-control eating. Nat. Med. 2022;28(9):1791–1796. doi: 10.1038/s41591-022-01941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.P. Magnetoencephalography: basic principles. Ann. Indian Acad. Neurol. 2014;17(Suppl. 1):S107. doi: 10.4103/0972-2327.128676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son B.-c., Shon Y.M., Choi J.-g., Kim J., Ha S.-w., Kim S.-H., Lee S.-h. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact. Funct. Neurosurg. 2016;94(3):187–197. doi: 10.1159/000446611. [DOI] [PubMed] [Google Scholar]
- Stefani A., Peppe A., Pierantozzi M., Galati S., Moschella V., Stanzione P., Mazzone P. Multi-target strategy for Parkinsonian patients: the role of deep brain stimulation in the centromedian–parafascicularis complex. Brain Res. Bull. 2009;78(2–3):113–118. doi: 10.1016/j.brainresbull.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Štillová K., Jurák P., Chládek J., Chrastina J., Halámek J., Bočková M., Goldemundová S., Říha I., Rektor I. The role of anterior nuclei of the thalamus: a subcortical gate in memory processing: an intracerebral recording study. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0140778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D., Chen H., Hu W., Zhou J., Feng T. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson's disease: a meta-analysis of controlled trials. Brain Stimul.: Basic, Translational, and Clinical Research in Neuromodulation. 2019;12(2):389–390. [Google Scholar]
- Sun F.T., Morrell M.J. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expet Rev. Med. Dev. 2014;11(6):563–572. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- Swann N.C., de Hemptinne C., Thompson M.C., Miocinovic S., Miller A.M., Ostrem J.L., Chizeck H.J., Starr P.A. Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J. Neural. Eng. 2018;15(4) doi: 10.1088/1741-2552/aabc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Buentjen L., Voges J., Schmitt F.C., Zaehle T., Kam J.W., Kaufmann J., Heinze H.-J., Hinrichs H., Knight R.T. The role of the anterior nuclei of the thalamus in human memory processing. Neurosci. Biobehav. Rev. 2021;126:146–158. doi: 10.1016/j.neubiorev.2021.02.046. [DOI] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Lee H., Rampp S., Zaehle T., Buentjen L., Voges J., Holtkamp M., Hinrichs H., Heinze H.-J., Schmitt F.C. Thalamic interictal epileptiform discharges in deep brain stimulated epilepsy patients. J. Neurol. 2016;263(10):2120–2126. doi: 10.1007/s00415-016-8246-5. [DOI] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Zaehle T., Voges J., Schmitt F.C., Buentjen L., Borchardt V., Walter M., Hinrichs H., Heinze H.-J., Rugg M.D. Anterior thalamic high frequency band activity is coupled with theta oscillations at rest. Front. Hum. Neurosci. 2017;11:358. doi: 10.3389/fnhum.2017.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Zaehle T., Voges J., Schmitt F.C., Buentjen L., Kopitzki K., Esslinger C., Hinrichs H., Heinze H.-J., Knight R.T., Richardson-Klavehn A. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. Elife. 2014;3 doi: 10.7554/elife.05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Zaehle T., Voges J., Schmitt F.C., Buentjen L., Kopitzki K., Richardson-Klavehn A., Hinrichs H., Heinze H.-J., Knight R.T. Pre-stimulus thalamic theta power predicts human memory formation. Neuroimage. 2016;138:100–108. doi: 10.1016/j.neuroimage.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Taube J.S. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 2007;30(1):181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Topalovic U., Aghajan Z.M., Villaroman D., Hiller S., Christov-Moore L., Wishard T.J., Stangl M., Hasulak N.R., Inman C.S., Fields T.A. Wireless programmable recording and stimulation of deep brain activity in freely moving humans. Neuron. 2020;108(2):322–334. doi: 10.1016/j.neuron.2020.08.021. e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E., Kumar S.S., Chaitanya G., Riley K., Balasubramanian K., Pati S. Machine learning approach to detect focal-onset seizures in the human anterior nucleus of the thalamus. J. Neural. Eng. 2020;17(6) doi: 10.1088/1741-2552/abc1b7. [DOI] [PubMed] [Google Scholar]
- Tröster A.I., Meador K.J., Irwin C.P., Fisher R.S. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. 2017;45:133–141. doi: 10.1016/j.seizure.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Tsanov M., Chah E., Vann S.D., Reilly R.B., Erichsen J.T., Aggleton J.P., O'Mara S.M. Theta-modulated head direction cells in the rat anterior thalamus. J. Neurosci. 2011;31(26):9489–9502. doi: 10.1523/jneurosci.0353-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach G., Kantak P., Jacobs J., Kahana M., Pfeiffer B.E., Sperling M., Lega B. Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proc. Natl. Acad. Sci. USA. 2020;117(45):28463–28474. doi: 10.1073/pnas.2013250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentín A., García Navarrete E., Chelvarajah R., Torres C., Navas M., Vico L., Torres N., Pastor J., Selway R., Sola R.G. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54(10):1823–1833. doi: 10.1111/epi.12352. [DOI] [PubMed] [Google Scholar]
- Van der Werf Y.D., Scheltens P., Lindeboom J., Witter M.P., Uylings H.B., Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41(10):1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van Groen T., Wyss J.M. Projections from the anterodorsal and anteroveniral nucleus of the thalamus to the limbic cortex in the rat. J. Comp. Neurol. 1995;358(4):584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- Velasco F., Saucedo-Alvarado P.E., Reichrath A., Valdés-Quiroz H., Aguado-Carrillo G., Velasco A.L. Centromedian nucleus and epilepsy. J. Clin. Neurophysiol. 2021;38(6):485–493. doi: 10.1097/WNP.0000000000000735. [DOI] [PubMed] [Google Scholar]
- Velasco F., Velasco M., Jiménez F., Velasco A.L., Brito F., Rise M., Carrillo-Ruiz J.D. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47(2):295–305. doi: 10.1097/00006123-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Velasco F., Velasco M., Velasco A.L., Jimenez F. Effect of chronic electrical stimulation of the centromedian thalamic nuclei on various intractable seizure patterns: I. Clinical seizures and paroxysmal EEG activity. Epilepsia. 1993;34(6):1052–1064. doi: 10.1111/j.1528-1157.1993.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Velasco M., Velasco F., Velasco A.L., Luján M., del Mercado J.V. Epileptiform EEG activities of the centromedian thalamic nuclei in patients with intractable partial motor, complex partial, and generalized seizures. Epilepsia. 1989;30(3):295–306. doi: 10.1111/j.1528-1157.1989.tb05301.x. [DOI] [PubMed] [Google Scholar]
- Velisar A., Syrkin-Nikolau J., Blumenfeld Z., Trager M., Afzal M., Prabhakar V., Bronte-Stewart H. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019;12(4):868–876. doi: 10.1016/j.brs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Vertes R., Albo Z., Di Prisco G.V. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience. 2001;104(3):619–625. doi: 10.1016/s0306-4522(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Vetkas A., Fomenko A., Germann J., Sarica C., Iorio-Morin C., Samuel N., Yamamoto K., Milano V., Cheyuo C., Zemmar A. vol. 63. 2022. pp. 1885–1886. (Response: Deep Brain Stimulation Targets in Epilepsy: Systematic Review and Meta-Analysis of Anterior and Centromedian Thalamic Nuclei and hippocampus). [DOI] [PubMed] [Google Scholar]
- Vetkas A., Fomenko A., Germann J., Sarica C., Iorio‐Morin C., Samuel N., Yamamoto K., Milano V., Cheyuo C., Zemmar A. Deep brain stimulation targets in epilepsy: systematic review and meta‐analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia. 2022;63(3):513–524. doi: 10.1111/epi.17157. [DOI] [PubMed] [Google Scholar]
- Wirt R., Hyman J. Integrating spatial working memory and remote memory: interactions between the medial prefrontal cortex and Hippocampus. Brain Sci. 2017;7(12):43. doi: 10.3390/brainsci7040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S.P., Fields J.A., Lyons K.E., Pahwa R., Tröster A.I. Pulse width is associated with cognitive decline after thalamic stimulation for essential tremor. Park. Relat. Disord. 2003;9(5):295–300. doi: 10.1016/s1353-8020(03)00014-2. [DOI] [PubMed] [Google Scholar]
- Wright N.F., Erichsen J.T., Vann S.D., O'Mara S.M., Aggleton J.P. Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. J. Comp. Neurol. 2010;518(12):2334–2354. doi: 10.1002/cne.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Misaki M., Phillips R., Wong C.K., Bodurka J. Real‐time fMRI neurofeedback of the mediodorsal and anterior thalamus enhances correlation between thalamic BOLD activity and alpha EEG rhythm. Hum. Brain Mapp. 2018;39(2):1024–1042. doi: 10.1002/hbm.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.