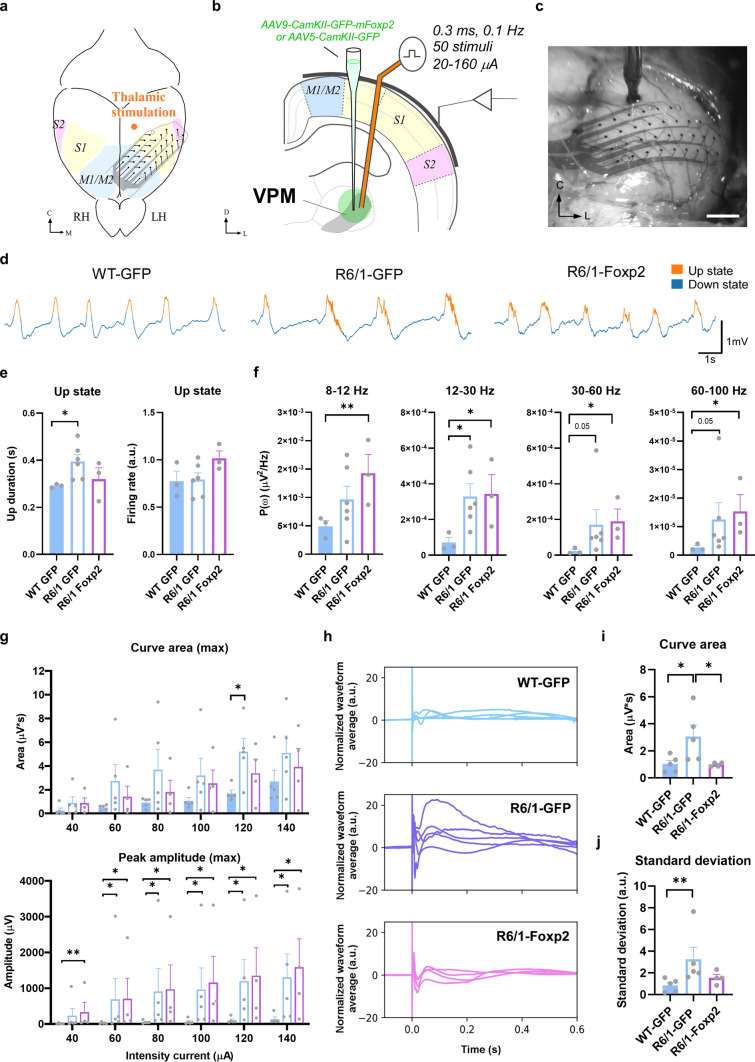
Fig. 6.
Effects on spontaneous activity and thalamo-cortical evoked responses upon Foxp2 levels recovery in the R6/1 mice thalamus. a Schematic representation of the experimental setup in a top view of the mouse brain and b a coronal section. The bipolar stimulation electrode was placed in VPM thalamic nucleus to elicit cortical responses in anesthetized mice. LFP were recorded from different motor cortex (M1/M2) and the primary (S1) and secondary (S2) somatosensory cortical areas through a superficial 32-channels MEA. c Microphotograph showing the array placed in the cortical surface of left hemisphere during an experiment. Scale bar: 500 µm. d Representative raw traces of the local field potentials (LFP) in the three experimental groups (16-week-old WT-GFP, R6/1-GFP and R6/1-Foxp2 groups of mice) are shown. Up and down events are colored in orange and blue, respectively. e Quantification of the Up states mean duration (in seconds; left panel) and the mean firing rate (arbitrary units; right panel) during the Up states. f Averaged power spectral density (PSD) over the z-scored normalized LFP of oscillatory activity at different frequency bands (alpha, 8–12 Hz; beta, 12–30 Hz; low-gamma, 30–60 Hz; high-gamma, 60–100 Hz) in WT-GFP, R6/1-GFP and R6/1-Foxp2 mice. g The evoked responses in the cortex in the first 50 ms after the stimulation. The responses evoked in the 32 channels were quantified, and the maximum area under the curve (upper panel) and maximum peak amplitude (lower panel) of the first wave are shown for the range of 40–140 µA intensity currents. h Long-lasting baseline z-score normalized evoked responses (in a.u.) after thalamic stimulation at 160 µA in WT-GFP (blue; upper panel), R6/1-GFP (purple; middle panel) and R6/1-Foxp2 (pink, lower panel) mice. Solid lines indicate the trial waveforms of each animal. i The area under the curve was quantified for a time-window of 600 ms post-stimuli using the 160 µA intensity current protocol for each experimental group. j The value distribution of the area under the curve of the normalized evoked responses in the 32 channels recorded was quantified as the standard deviation (in a.u.). Data are represented as mean ± SEM. Kruskal–Wallis test was used, *p < 0.05, **p < 0.01 compared with WT-GFP mice. RH right hemisphere, LH left hemisphere, M1/M2 motor areas 1 and 2, S1 sensory area, VPM ventral posteromedial nucleus, C center, L left