Abstract
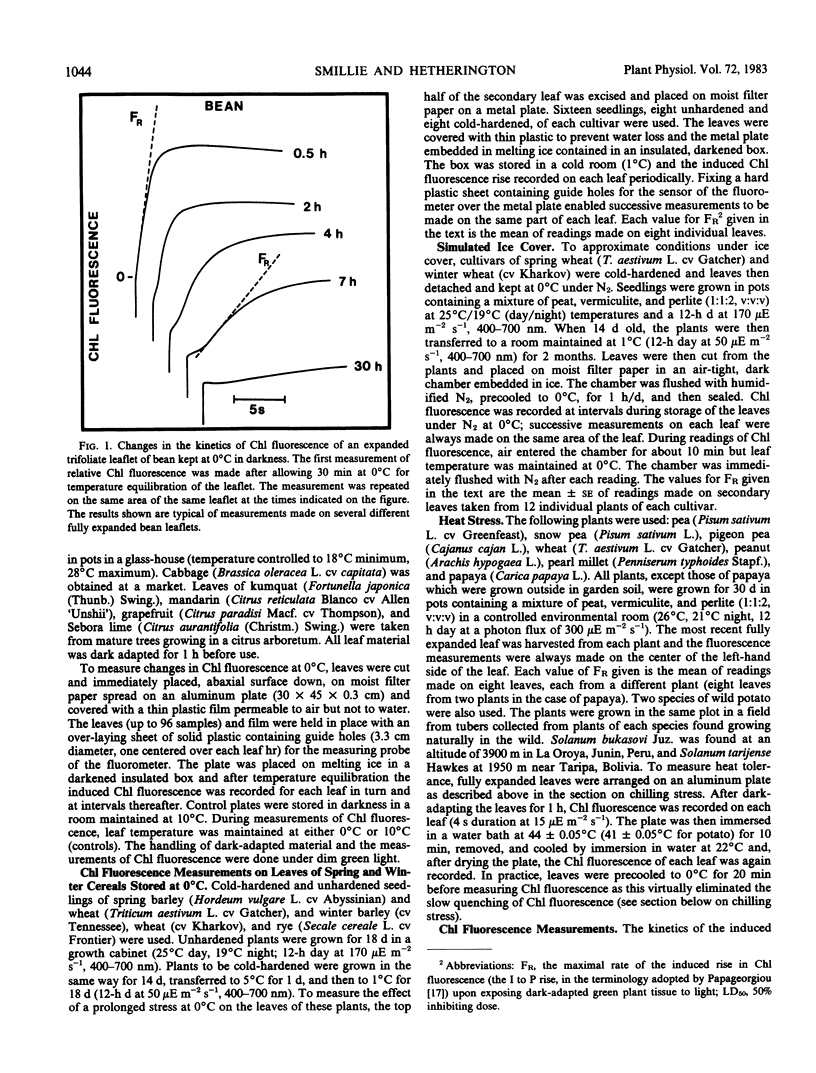
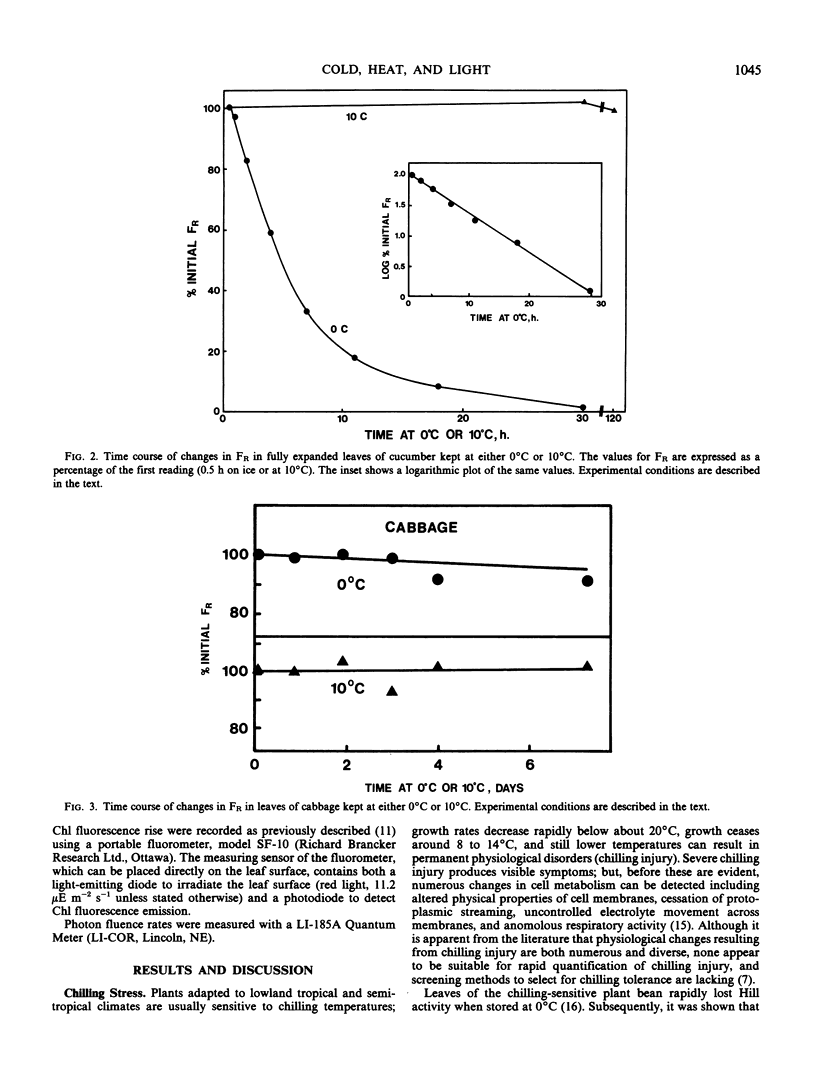
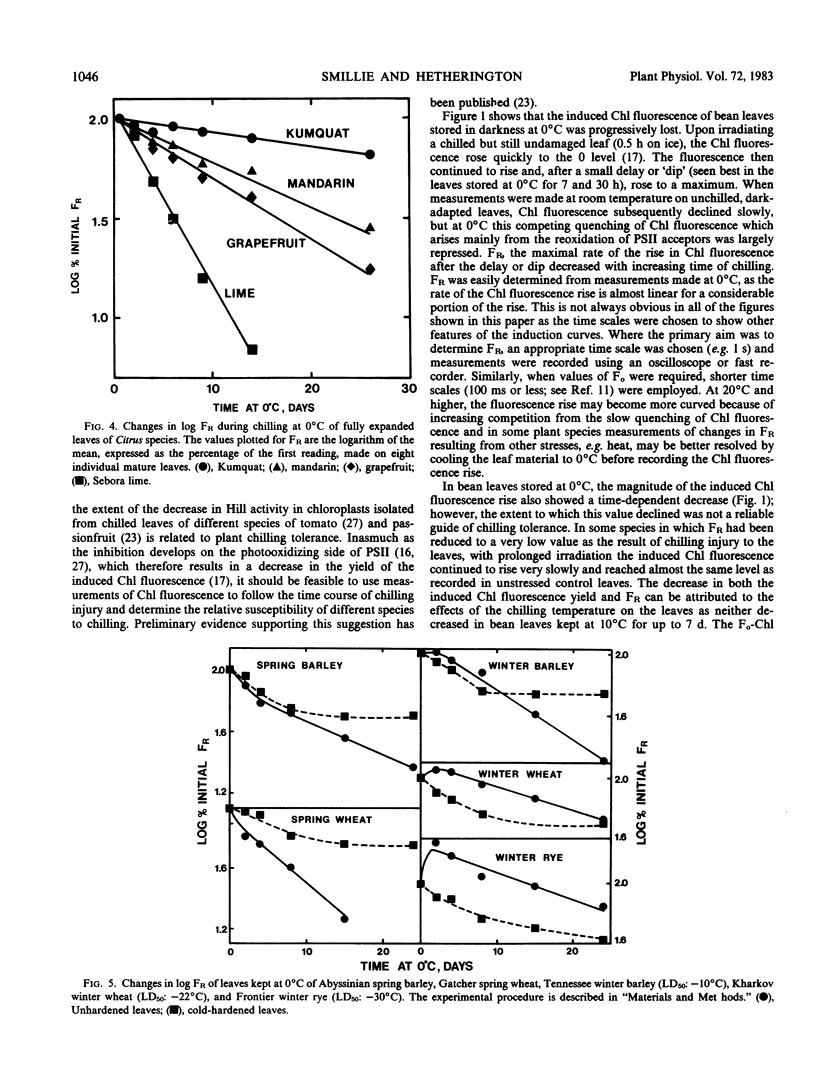
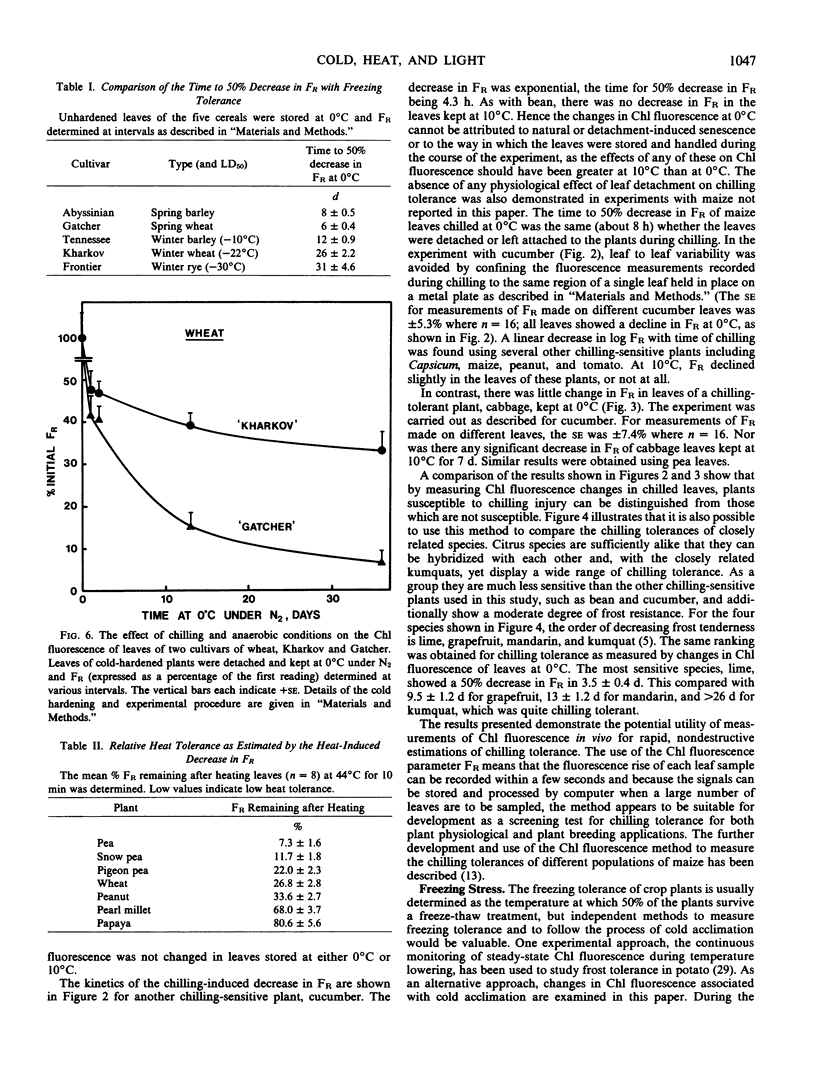
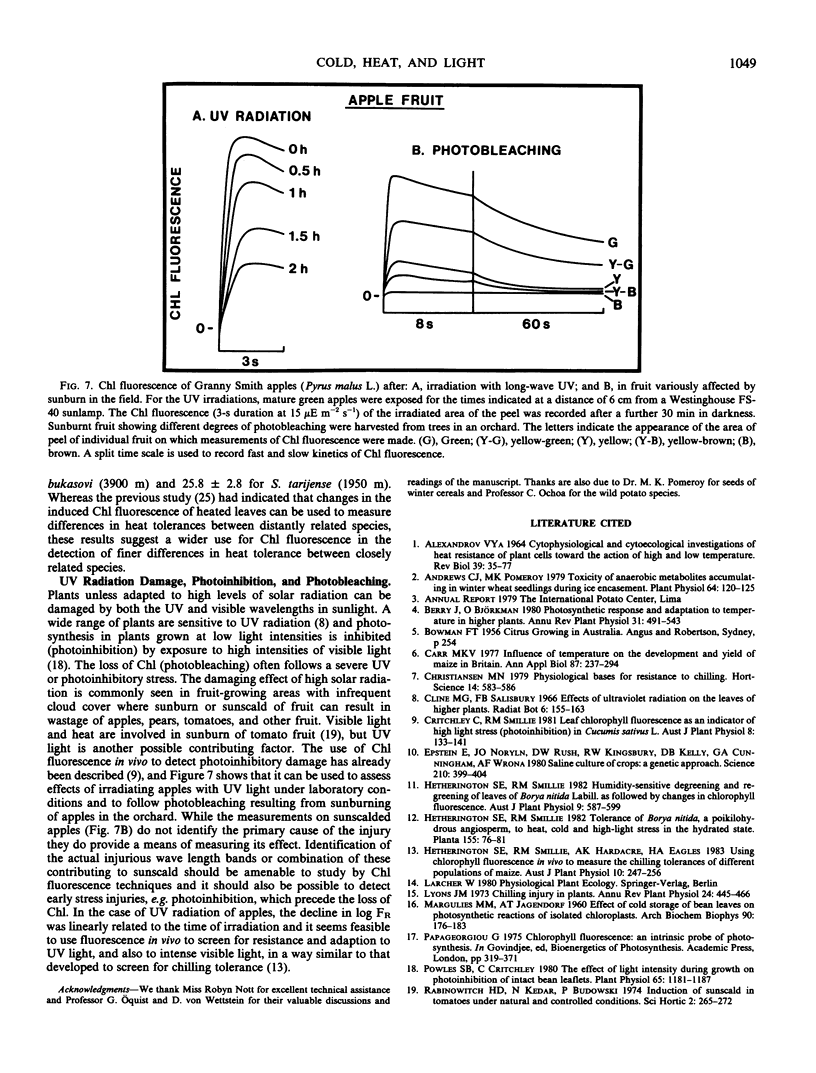
The proposition is examined that measurements of chlorophyll fluorescence in vivo can be used to monitor cellular injury caused by environmental stresses rapidly and nondestructively and to determine the relative stress tolerances of different species. Stress responses of leaf tissue were measured by FR, the maximal rate of the induced rise in chlorophyll fluorescence. The time taken for FR to decrease by 50% in leaves at 0°C was used as a measure of chilling tolerance. This value was 4.3 hours for chilling-sensitive cucumber. In contrast, FR decreased very slowly in cucumber leaves at 10°C or in chilling-tolerant cabbage leaves at 0°C. Long-term changes in FR of barley, wheat, and rye leaves kept at 0°C were different in frost-hardened and unhardened material and in the latter appeared to be correlated to plant frost tolerance. To simulate damage caused by a thick ice cover, wheat leaves were placed at 0°C under N2. Kharkov wheat, a variety tolerant of ice encapsulation, showed a slower decrease in FR than Gatcher, a spring wheat. Relative heat tolerance was also indicated by the decrease in FR in heated leaves while changes in vivo resulting from photoinhibition, ultraviolet radiation, and photobleaching can also be measured.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Pomeroy M. K. Toxicity of Anaerobic Metabolites Accumulating in Winter Wheat Seedlings during Ice Encasement. Plant Physiol. 1979 Jul;64(1):120–125. doi: 10.1104/pp.64.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Norlyn J. D., Rush D. W., Kingsbury R. W., Kelley D. B., Cunningham G. A., Wrona A. F. Saline culture of crops: a genetic approach. Science. 1980 Oct 24;210(4468):399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- MARGULIES M. M., JAGENDORF A. T. Effect of cold storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Arch Biochem Biophys. 1960 Oct;90:176–183. doi: 10.1016/0003-9861(60)90565-8. [DOI] [PubMed] [Google Scholar]
- Powles S. B., Critchley C. Effect of Light Intensity during Growth on Photoinhibition of Intact Attached Bean Leaflets. Plant Physiol. 1980 Jun;65(6):1181–1187. doi: 10.1104/pp.65.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Critchley C., Bain J. M., Nott R. Effect of growth temperature on chloroplast structure and activity in barley. Plant Physiol. 1978 Aug;62(2):191–196. doi: 10.1104/pp.62.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Nott R. Assay of chilling injury in wild and domestic tomatoes based on photosystem activity of the chilled leaves. Plant Physiol. 1979 May;63(5):796–801. doi: 10.1104/pp.63.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Nott R. Salt tolerance in crop plants monitored by chlorophyll fluorescence in vivo. Plant Physiol. 1982 Oct;70(4):1049–1054. doi: 10.1104/pp.70.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundbom E., Strand M., Hällgren J. E. Temperature-induced fluorescence changes : a screening method for frost tolerance of potato (solanum sp.). Plant Physiol. 1982 Nov;70(5):1299–1302. doi: 10.1104/pp.70.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]


