Abstract
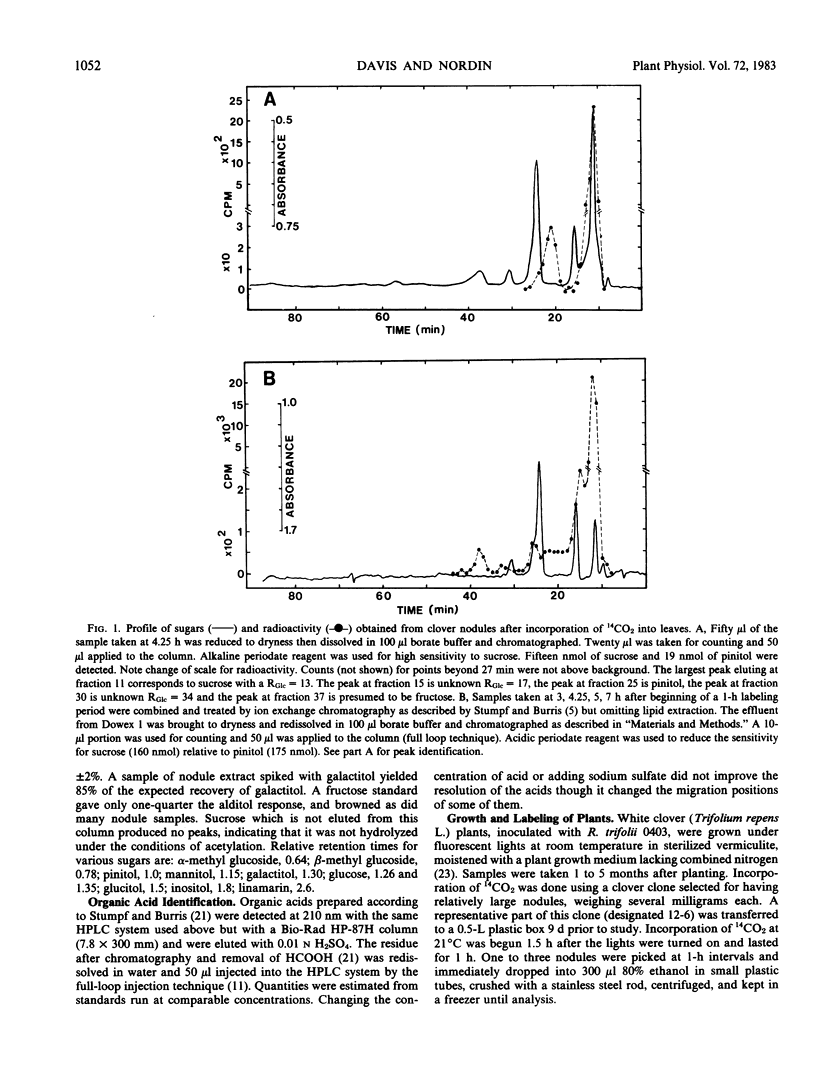
Major ethanol-soluble carbohydrate and organic acid constituents of white clover (Trifolium repens) have been identified by use of high-performance liquid chromatography and gas chromatography. In leaves, petioles, roots, and nodules, pinitol (3-O-methyl chiro-inositol) is the predominant sugar, with sucrose present in lower concentration. In leaves and petioles there are significant levels of α- and β-methyl glucosides, linamarin, glucose, and fructose. In the nodules glucose is rarely present at detectable levels. The concentration of pinitol is generally greater than 25 millimolar in each tissue examined whereas the level of sucrose varies depending on the time of day. Sucrose is the major sugar significantly labeled during 1 hour administration of 14CO2 and accounts for more than 99% of all the radioactivity detected in the nodules at early times. Between 3 and 7 hours after labeling, 6% of the radioactivity is found in the organic acids fraction and 5% in the basic fraction of nodules. Malonic acid does not appear to be present in unusually high concentrations in either leaves or nodules of white clover.
Full text
PDF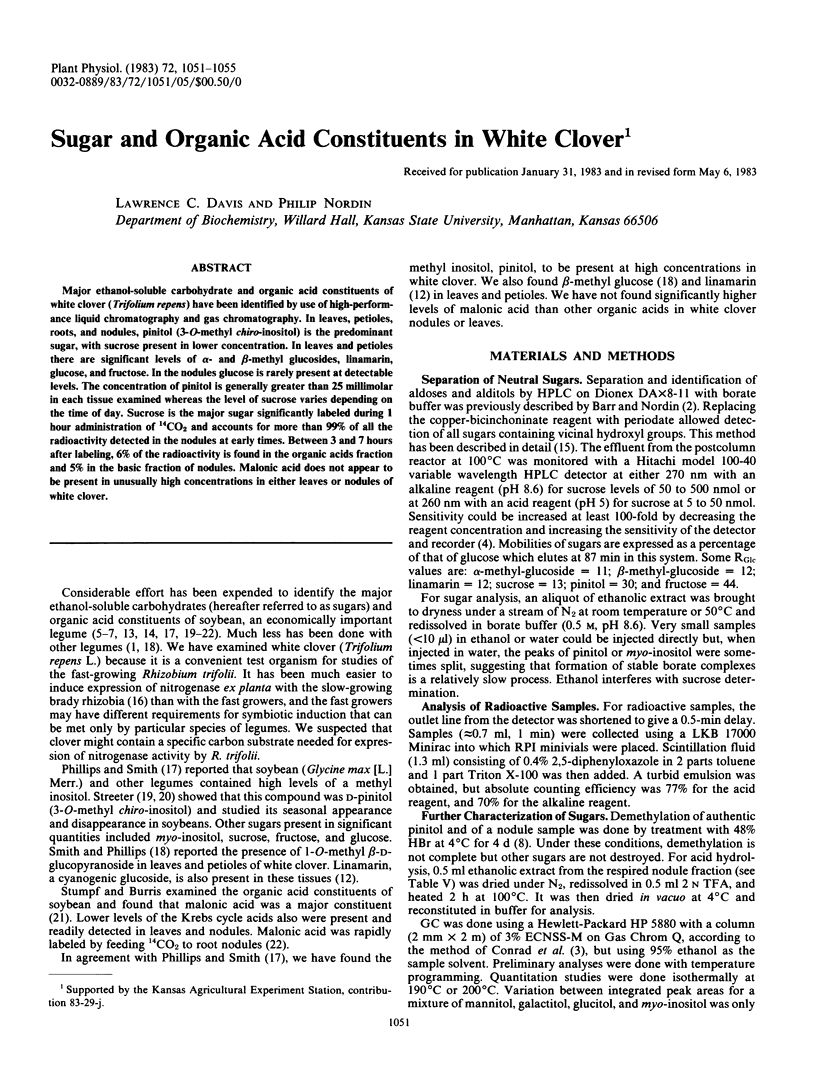


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr J., Nordin P. Microdetermination of neutral and amino sugars found in glycoproteins. Anal Biochem. 1980 Nov 1;108(2):313–319. doi: 10.1016/0003-2697(80)90591-6. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Ager-Johnson P., Woo M. L. Antibodies against the predominant glycosaminoglycan of the mammalian cornea, keratan sulfate-I. J Biol Chem. 1982 Jan 10;257(1):464–471. [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 Translocation in Soybean: II. Kinetics in the Leaf. Plant Physiol. 1970 Feb;45(2):114–118. doi: 10.1104/pp.45.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 translocation in soybean: I. Kinetics in the stem. Plant Physiol. 1970 Feb;45(2):107–113. doi: 10.1104/pp.45.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage R. S., Aronoff S. Translocation III. Experiments with Carbon 14, Chlorine 36, and Hydrogen 3. Plant Physiol. 1960 Jan;35(1):53–64. doi: 10.1104/pp.35.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. D., Clauss H., Mortimer D. C., Gorham P. R. Selective translocation of products of photosynthesis in soybean. Plant Physiol. 1961 Sep;36(5):581–588. doi: 10.1104/pp.36.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrates in Soybean Nodules: II. DISTRIBUTION OF COMPOUNDS IN SEEDLINGS DURING THE ONSET OF NITROGEN FIXATION. Plant Physiol. 1980 Sep;66(3):471–476. doi: 10.1104/pp.66.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. A micromethod for the purification and quantification of organic acids of the tricarboxylic acid cycle in plant tissues. Anal Biochem. 1979 May;95(1):311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Biosynthesis of malonate in roots of soybean seedlings. Plant Physiol. 1981 Nov;68(5):992–995. doi: 10.1104/pp.68.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]


