Abstract
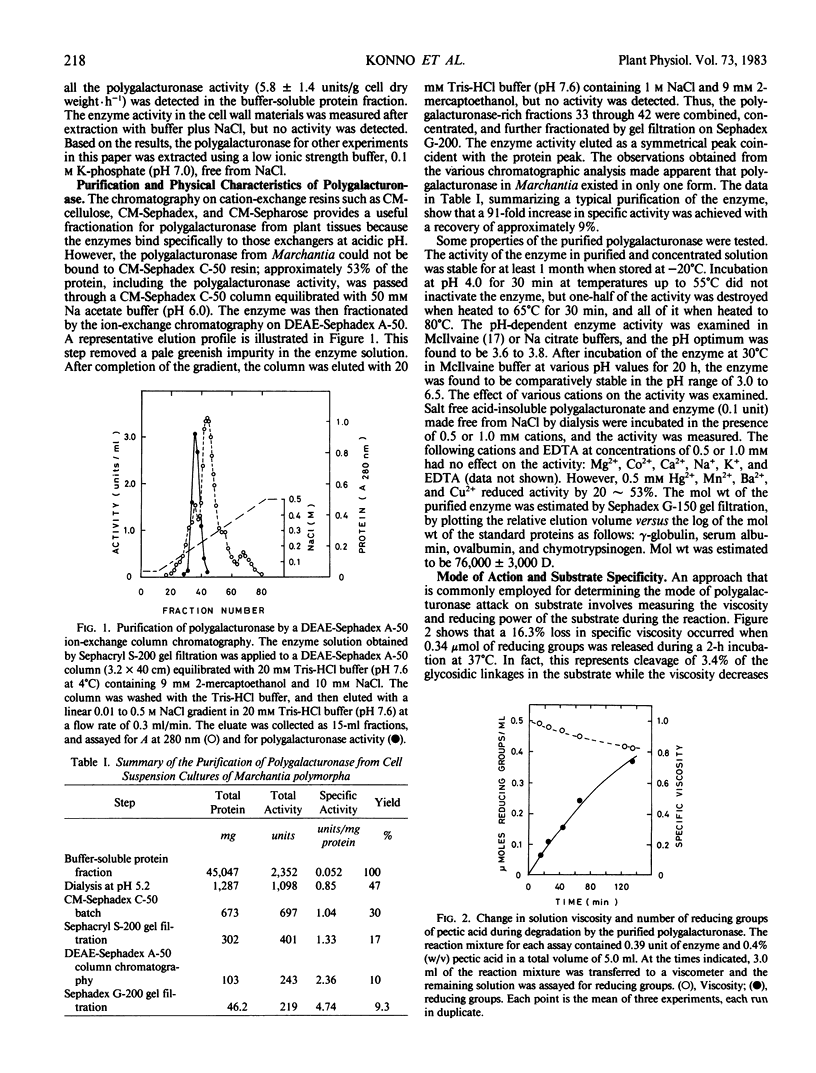
Polygalacturonase was isolated from cell suspension cultures of a thalloid liverwort, Marchantia polymorpha. The enzyme in the `buffer-soluble' protein fraction was dialyzed at pH 5.2 and further purified 91-fold by a combination of chromatographic techniques including CM-Sephadex, Sephacryl S-200, DEAE-Sephadex, and Sephadex G-200. The purified enzyme had an optimum activity in the pH range at 3.6 to 3.8 and molecular weight of 76,000 daltons, and its activity was not stimulated by cations. The enzyme was identified as an exohydrolase from viscometric data and chromatographic analysis of the reaction products.
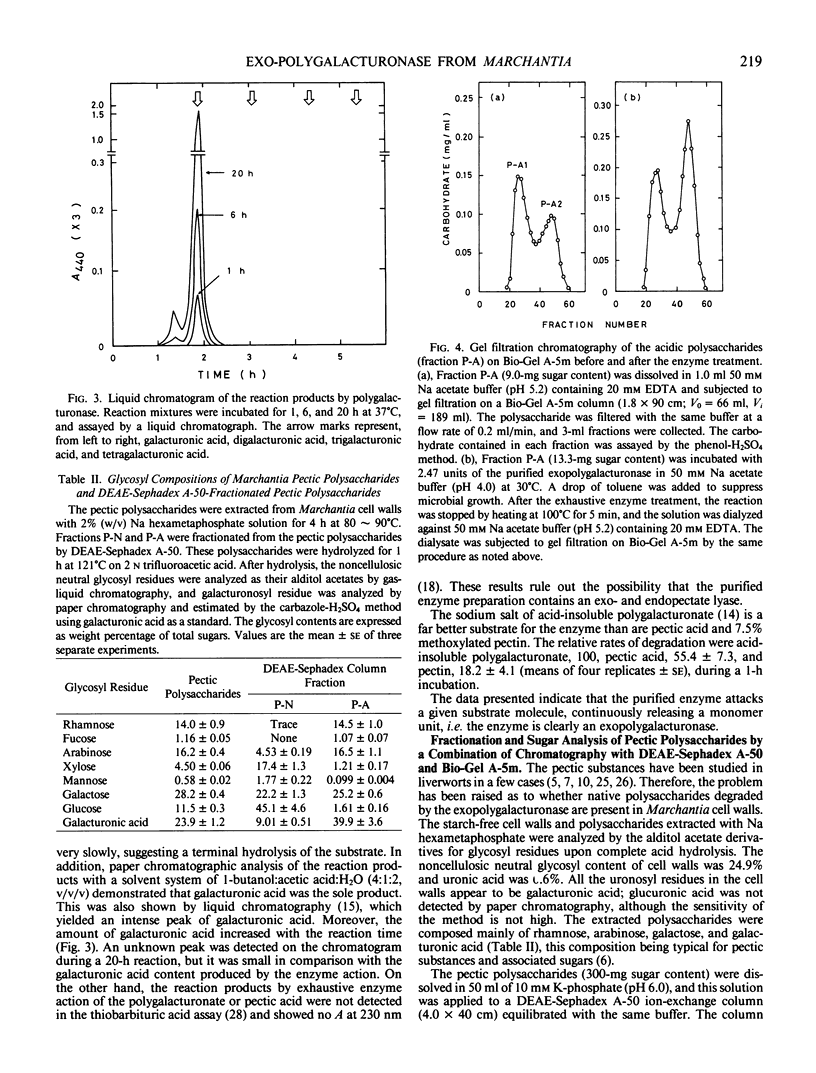
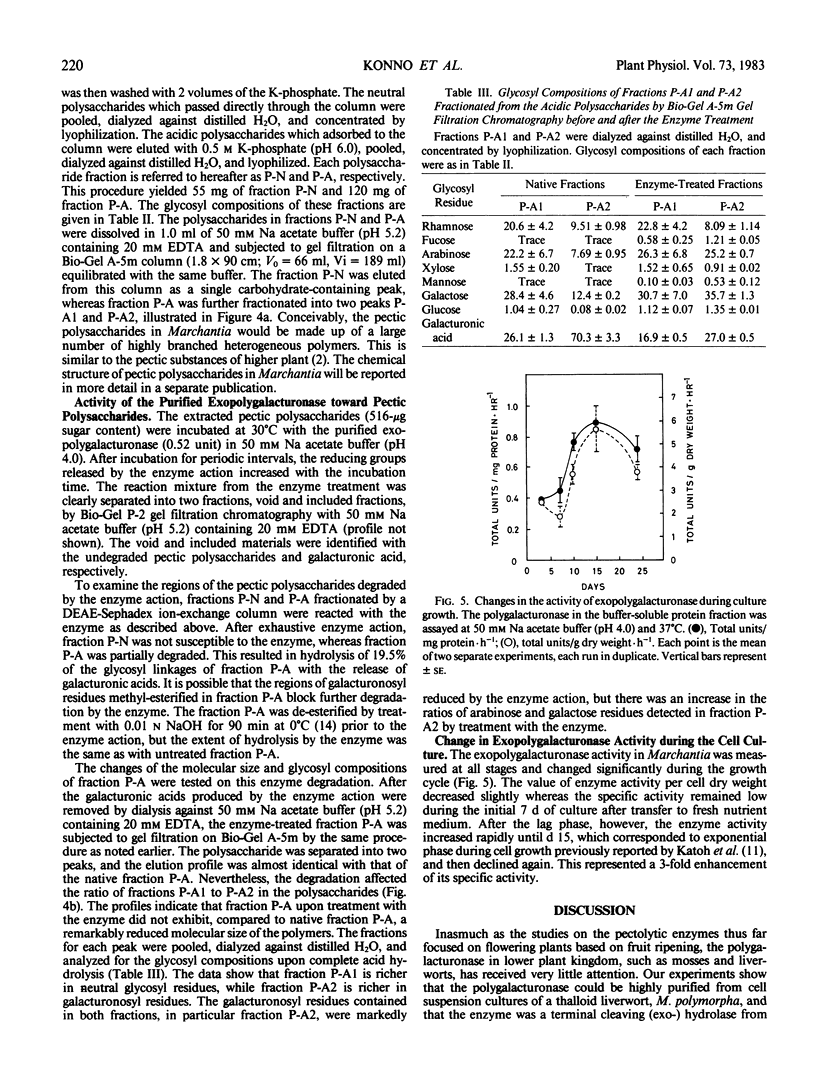
The polysaccharides extracted from the Marchantia cell walls with 2% (w/v) Na hexametaphosphate solution were separated into two fractions, neutral polysaccharides (fraction P-N) and acidic polysaccharides (fraction P-A) by a DEAE-Sephadex column. The fraction P-N was not susceptible to the purified exopolygalacturonase, whereas fraction P-A was partially degraded. This resulted in hydrolysis of 19.5% of the glycosyl linkages of fraction P-A with the release of galacturonic acids. The specific activity of exopolygalacturonase increased during the growth cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Konno H., Yamasaki Y. Studies on the Pectic Substances of Plant Cell Walls: III. DEGRADATION OF CARROT ROOT CELL WALLS BY ENDOPECTATE LYASE PURIFIED FROM ERWINIA AROIDEAE. Plant Physiol. 1982 Apr;69(4):864–868. doi: 10.1104/pp.69.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Occurrence and properties of polygalacturonase in Avena and other plants. Plant Physiol. 1977 Oct;60(4):548–553. doi: 10.1104/pp.60.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Separation and characterization of endopolygalacturonase and exopolygalacturonase from peaches. Plant Physiol. 1973 Sep;52(3):252–256. doi: 10.1104/pp.52.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


