Abstract
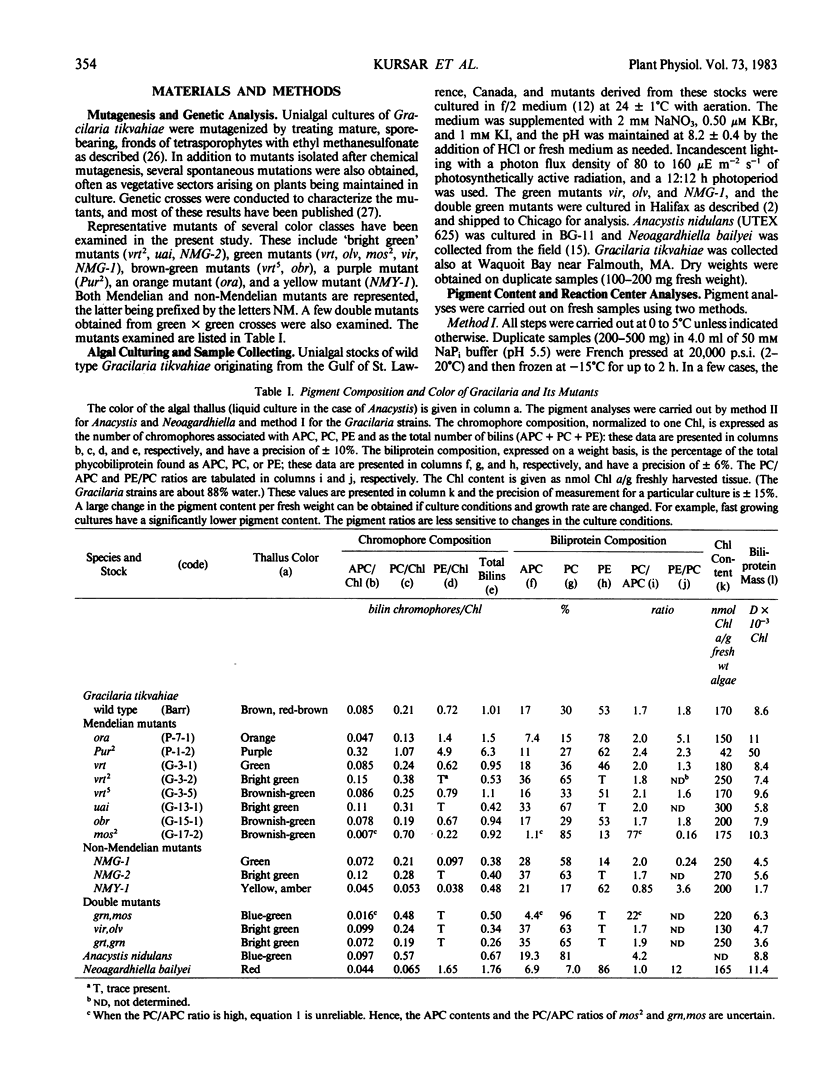
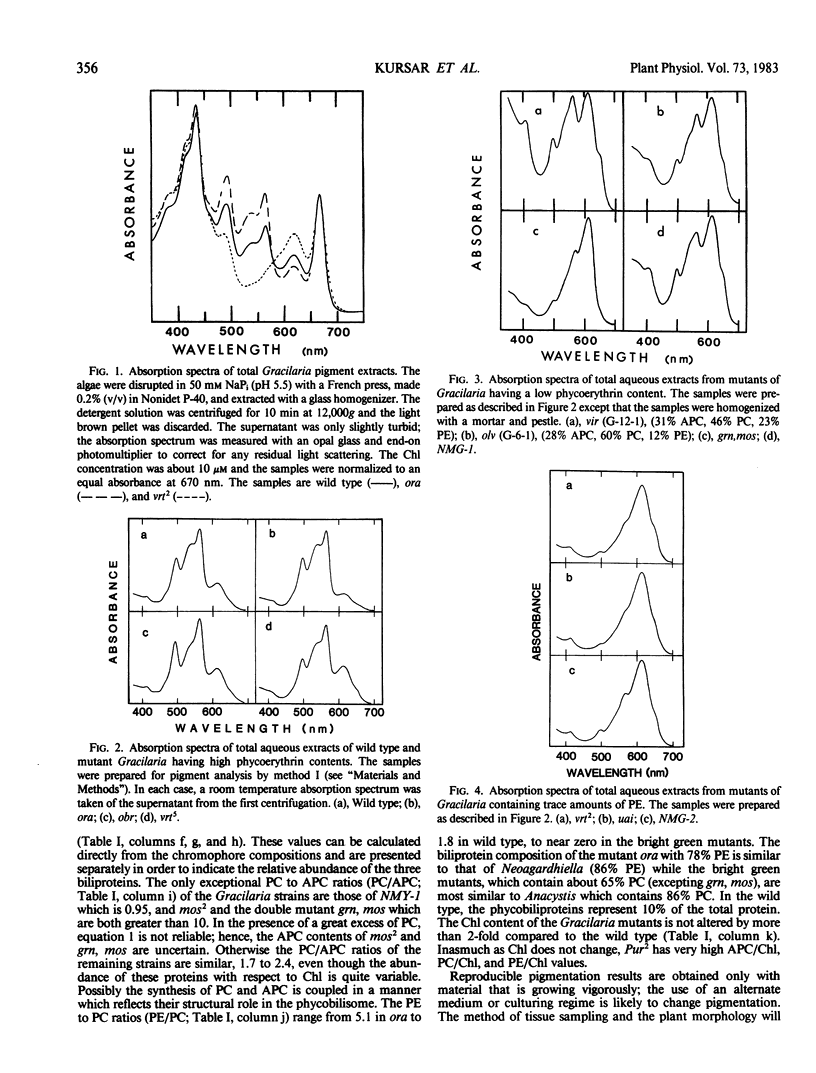
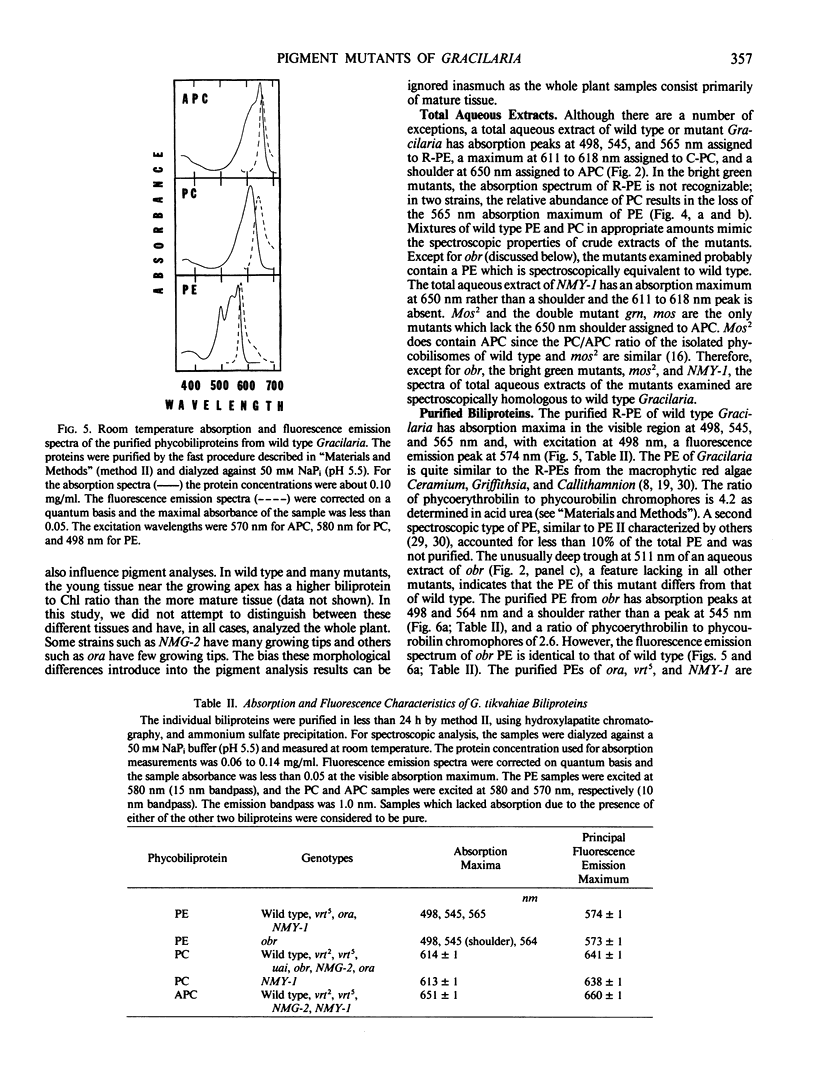
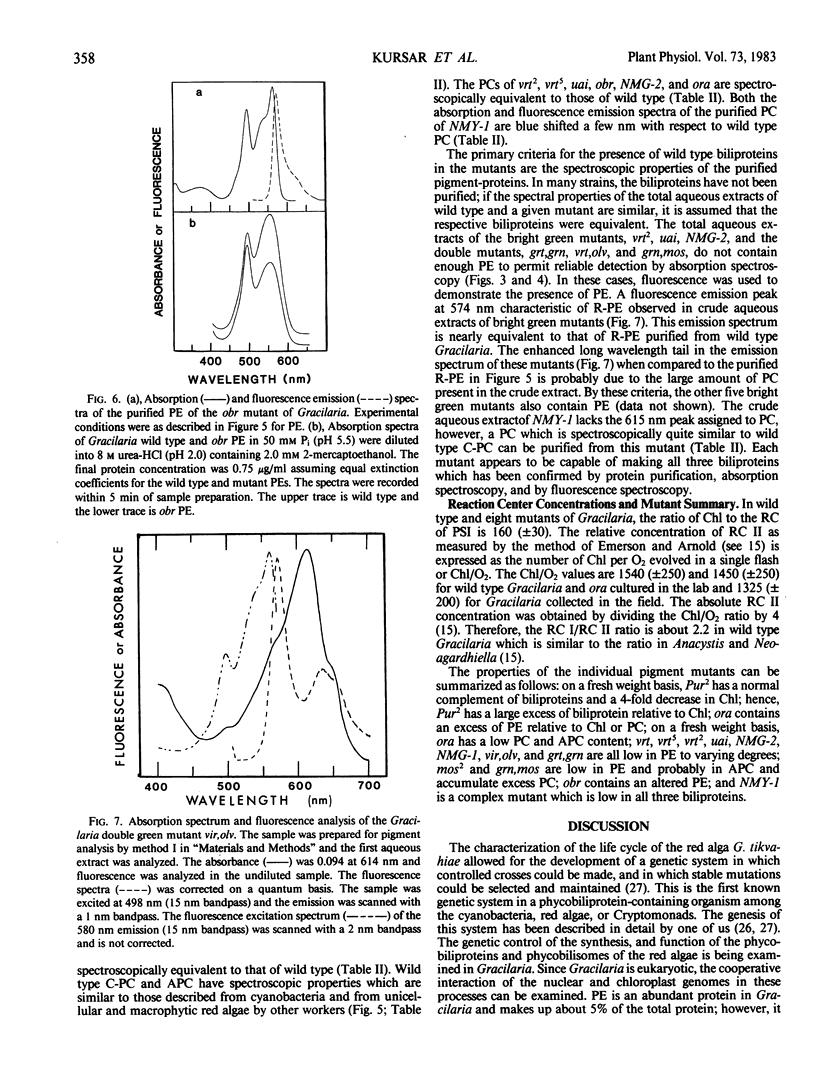
Wild type Gracilaria tikvahiae, a macrophytic red alga, and fourteen genetically characterized pigment mutants were analyzed for their biliprotein and chlorophyll contents. The same three biliproteins, phycoerythrin, phycocyanin, and allophycocyanin, which are found in the wild type are found in all the Mendelian and non-Mendelian mutants examined. Some mutants overproduce R-phycoerythrin while others possess only traces of phycobiliprotein; however, no phycoerythrin minus mutants were found. Two of the mutants are unique; one overproduces phycocyanin relative to allophycocyanin while the nuclear mutant obr synthesizes a phycoerythrin which is spectroscopically distinct from the R-phycoerythrin of the wild type. The phycoerythrin of obr lacks the typical absorption peak at 545 nanometers characteristic of R-phycoerythrin and possesses a phycoerythrobilin to phycourobilin chromophore ratio of 2.6 in contrast to a ratio of 4.2 found in the wild type. Such a lesion provides evidence for the role of nuclear genes in phycoerythrin synthesis. In addition, comparisons are made of the pigment compositions of the Gracilaria strains with those of Neoagardhiella bailyei, a macrophytic red alga which has a high phycoerythrin content, and Anacystis nidulans, a cyanobacterium which lacks phycoerythrin. The mutants described here should prove useful in the study of the genetic control of phycobiliprotein synthesis and phycobilisome structure and assembly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns D. S. Immunochemistry of biliproteins. Plant Physiol. 1967 Nov;42(11):1569–1586. doi: 10.1104/pp.42.11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Offner G. D., Ehrhardt M. M., Troxler R. F. Phycobilin-apoprotein linkages in the alpha and beta subunits of phycocyanin from the unicellular rhodophyte, Cyanidium caldarium. Amino acid sequences of 35S-labeled chromopeptides. J Biol Chem. 1979 Aug 25;254(16):7803–7811. [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Apell G. S., Hixson C. S., Bryant D. A., Rimon S., Brown D. M. Biliproteins of cyanobacteria and Rhodophyta: Homologous family of photosynthetic accessory pigments. Proc Natl Acad Sci U S A. 1976 Feb;73(2):428–431. doi: 10.1073/pnas.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem. 1975 Jul 25;250(14):5487–5495. [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- Kursar T. A., Alberte R. S. Photosynthetic Unit Organization in a Red Alga : Relationships between Light-Harvesting Pigments and Reaction Centers. Plant Physiol. 1983 Jun;72(2):409–414. doi: 10.1104/pp.72.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar T. A., van der Meer J., Alberte R. S. Light-Harvesting System of the Red Alga Gracilaria tikvahiae: II. Phycobilisome Characteristics of Pigment Mutants. Plant Physiol. 1983 Oct;73(2):361–369. doi: 10.1104/pp.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdowall F. D., Bednar T., Rosenberg A. Conformation dependence of intramolecular energy transfer in phycoerythrin. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1356–1363. doi: 10.1073/pnas.59.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCARRA P. PURIFICATION AND N-TERMINAL ANALYSES OF ALGAL BILIPROTEINS. Biochem J. 1965 Jan;94:171–174. doi: 10.1042/bj0940171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N., Spencer K. G., West J. A. Phycoerythrins of the Red Alga Callithamnion: VARIATION IN PHYCOERYTHROBILIN AND PHYCOUROBILIN CONTENT. Plant Physiol. 1981 Aug;68(2):482–488. doi: 10.1104/pp.68.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde H. H. The natural occurrence in red algae of two phycoerythrins with different molecular weights and spectral properties. Biochim Biophys Acta. 1973 Apr 20;303(2):246–257. doi: 10.1016/0005-2795(73)90354-1. [DOI] [PubMed] [Google Scholar]


