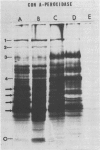
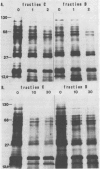
Abstract
Two fractions enriched in plasma membrane derived from suspension-cultured carrot (Daucus carota L.) cells were examined to determine if they differed from each other either in physical nature or in orientation. Parameters studied included the protein composition of purified membranes derived from trypsinized and nontrypsinized protoplasts as well as from trypsinized purified plasma membranes, the effect of inhibitors and membrane perturbants on ATPase activity, the binding of [acetyl-14C]concanavalin A to purified membrane fractions, and the competitive removal of [acetyl-14C]concanavalin A from purified membranes derived from [acetyl-14C]concanavalin A-labeled protoplasts. One fraction (at density of 1.102 grams per cubic centimeter on Renografin gradients) appears to be a mixed population of `tightly' sealed vesicles with the majority being rightside-out vesicles of plasma membrane, and the other fraction (density 1.128 grams per cubic centimeter) apparently is a population of predominantly `leaky' vesicles and/or nonvesicular fragments of plasma membrane, a large portion of which appear to be `leaky' inside-out vesicles. In addition, it is shown that plasma membrane-enriched fractions can be distinguished from cellular endomembranes on the basis of protein and glycoprotein composition.
Full text
PDF



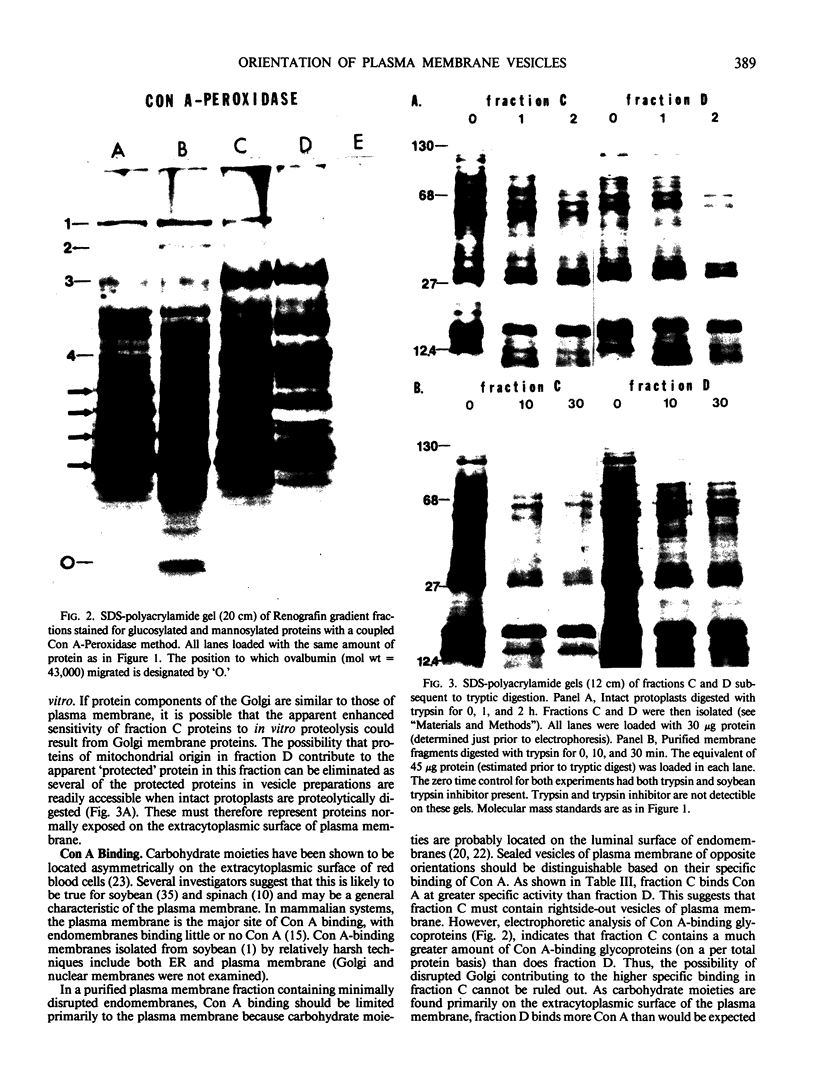


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz R. L., Travis R. L. Characterization and quantitation of concanavalin a binding by plasma membrane enriched fractions from soybean root. Plant Physiol. 1981 Nov;68(5):1014–1019. doi: 10.1104/pp.68.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booz M. L., Travis R. L. Electrophoretic comparison of polypeptides from enriched plasma membrane fractions from developing soybean roots. Plant Physiol. 1980 Dec;66(6):1037–1043. doi: 10.1104/pp.66.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Ruesink A. W. Isolation and Characterization of Concanavalin A-labeled Plasma Membranes of Carrot Protoplasts. Plant Physiol. 1979 Dec;64(6):1005–1011. doi: 10.1104/pp.64.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Resh M. D., Guidotti G. Vanadate inhibits the red cell (Na+, K+) ATPase from the cytoplasmic side. Nature. 1978 Apr 6;272(5653):552–554. doi: 10.1038/272552a0. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Burke L. L., Spanswick R. M. Characterization of a partially purified adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1981 Jan;67(1):59–63. doi: 10.1104/pp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Hugenholtz J., Hong J. S., Kaback H. R. ATP-driven active transport in right-side-out bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3446–3449. doi: 10.1073/pnas.78.6.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Franke W. W., Kartenbeck J. Concanavalin A binding by isolated plasma membranes and endomembranes from liver and mammary gland. FEBS Lett. 1974 Aug 30;44(3):274–278. doi: 10.1016/0014-5793(74)81156-7. [DOI] [PubMed] [Google Scholar]
- Kringstad R., Kenyon W. H., Black C. C. The rapid isolation of vacuoles from leaves of crassulacean Acid metabolism plants. Plant Physiol. 1980 Sep;66(3):379–382. doi: 10.1104/pp.66.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- Mellor R. B., Krusius T., Lord J. M. Analysis of glycoconjugate saccharides in organelles isolated from castor bean endosperm. Plant Physiol. 1980 Jun;65(6):1073–1075. doi: 10.1104/pp.65.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish R. W., Schmidlin S., Müller U. The effects of proteases on proteins and glycoproteins of Dictyostelium discoideum plasma membranes. Exp Cell Res. 1977 Dec;110(2):267–276. doi: 10.1016/0014-4827(77)90292-0. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Characterization of ATPase activity associated with corn leaf plasma membranes. Plant Physiol. 1981 Sep;68(3):521–526. doi: 10.1104/pp.68.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Labeling and isolation of plasma membranes from corn leaf protoplasts. Plant Physiol. 1980 Jun;65(6):1053–1057. doi: 10.1104/pp.65.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Steady-state kinetic study of vanadate-induced inhibition of ciliary dynein adenosinetriphosphatase activity from Tetrahymena. Biochemistry. 1981 Jul 21;20(15):4347–4354. doi: 10.1021/bi00518a018. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]