Abstract
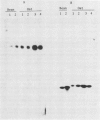
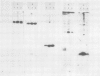
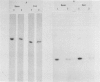
Antibodies against the individual subunits of protein complexes in the chloroplast membranes were used to follow the amounts of these polypeptides during foliar senescence. No change was found in the amount of polypeptides of photosystem I reaction center and the chloroplast coupling factor during senescence of oat (Avena sativa L.) and bean (Phaseolus vulgaris L.) leaves. A significant decrease in the amount of the different components of the cytochrome b6-f complex was detected. This change may account for the decrease in the rate of electron transport, which might be the rate limiting step of photosynthesis in senescing leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Purification and properties of the photosystem I reaction center from chloroplasts. J Biol Chem. 1975 Apr 25;250(8):2783–2788. [PubMed] [Google Scholar]
- Camp P. J., Huber S. C., Burke J. J., Moreland D. E. Biochemical Changes that Occur during Senescence of Wheat Leaves : I. Basis for the Reduction of Photosynthesis. Plant Physiol. 1982 Dec;70(6):1641–1646. doi: 10.1104/pp.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. Changes in the abscisic acid content of oat leaves during senescence. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2050–2053. doi: 10.1073/pnas.77.4.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Gil R., Schaedle M. Functional and Structural Changes in Senescing Populus deltoides (Bartr.) Chloroplasts. Plant Physiol. 1973 Feb;51(2):245–249. doi: 10.1104/pp.51.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kamienietzky A., Nelson N. Preparation and properties of chloroplasts depleted of chloroplast coupling factor 1 by sodium bromide treatment. Plant Physiol. 1975 Feb;55(2):282–287. doi: 10.1104/pp.55.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]