Abstract
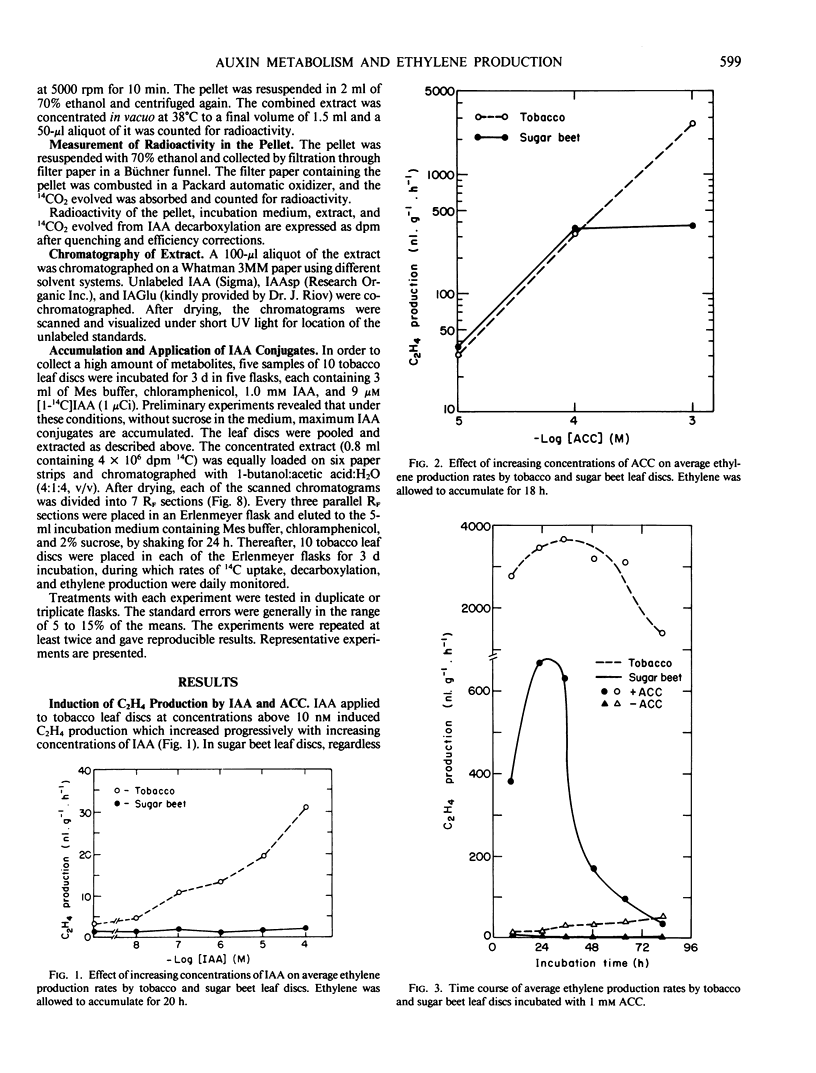
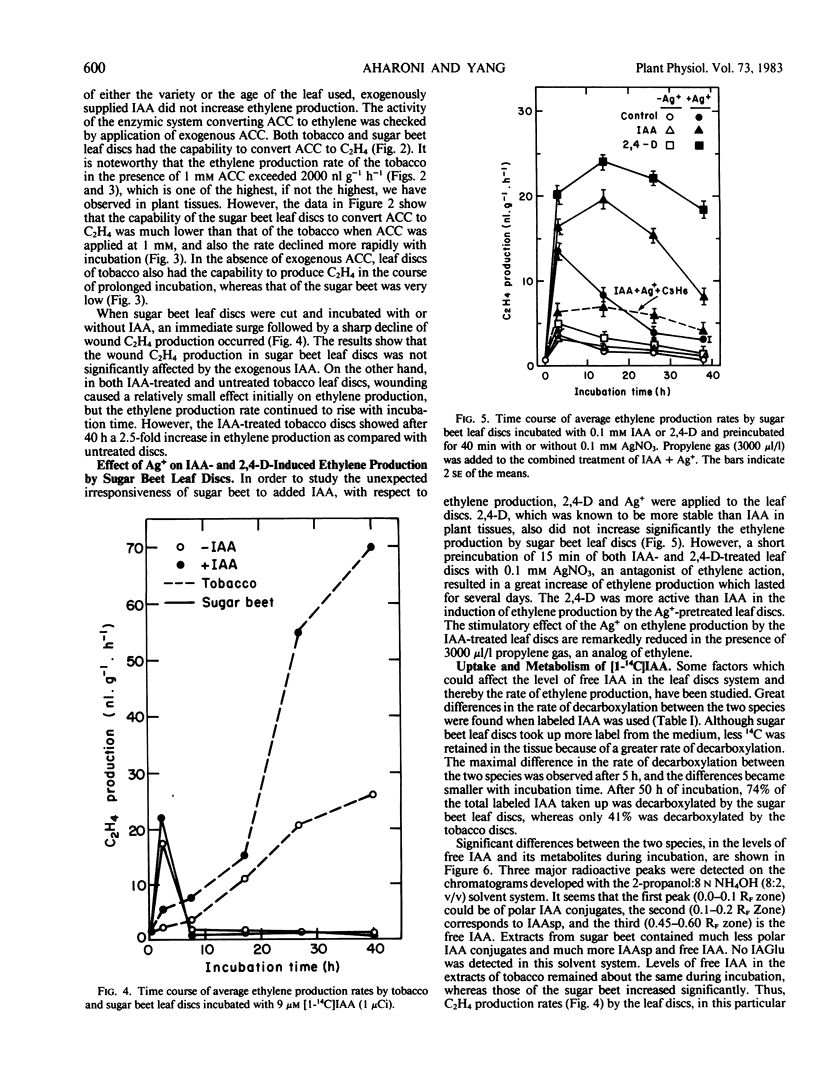
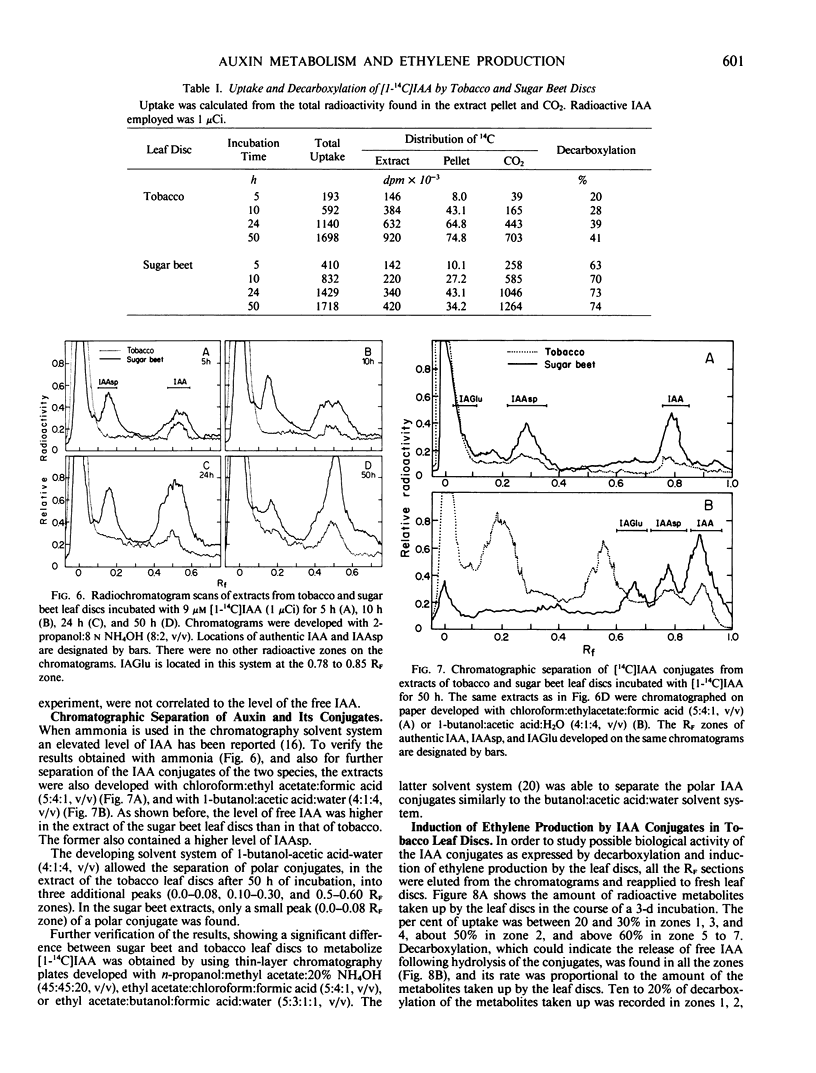
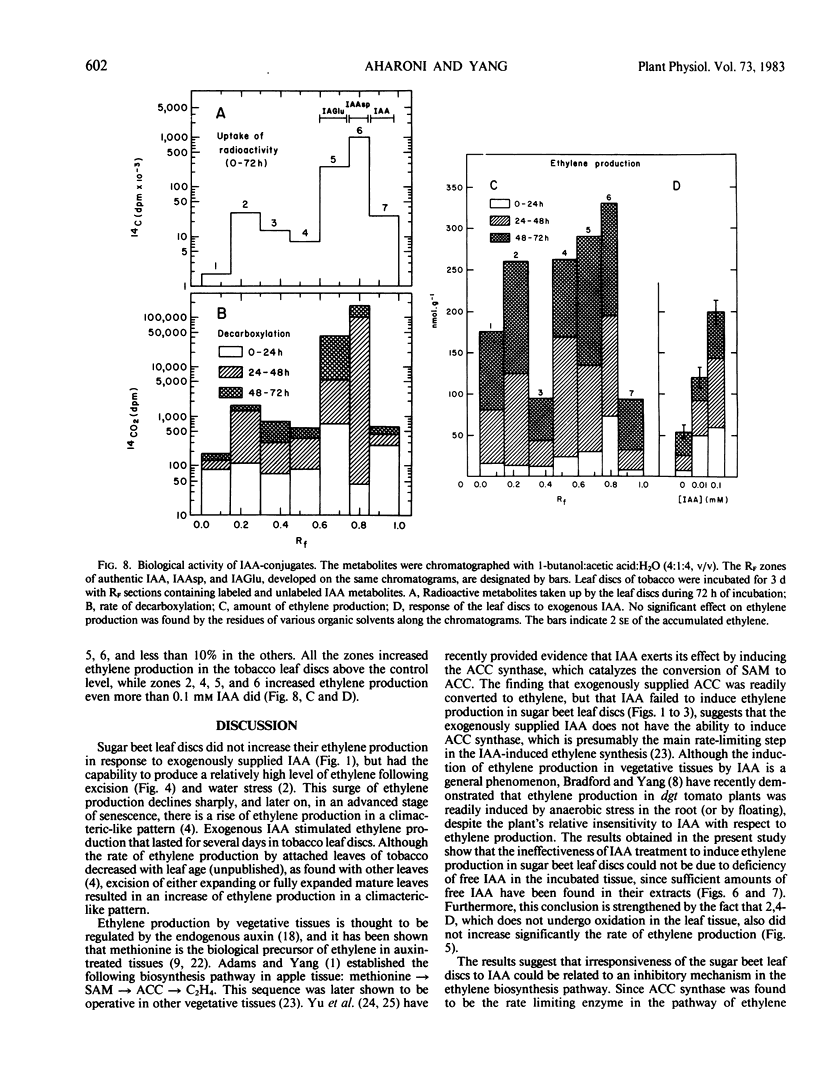
Exogenously supplied indole-3-acetic acid (IAA) stimulated ethylene production in tobacco (Nicotiana glauca) leaf discs but not in those of sugar beet (Beta vulgaris L.). The stimulatory effect of IAA in tobacco was relatively small during the first 24 hours of incubation but became greater during the next 24 hours. It was found that leaf discs of these two species metabolized [1-14C]IAA quite differently. The rate of decarboxylation in sugar beet discs was much higher than in tobacco. The latter contained much less free IAA but a markedly higher level of IAA conjugates. The major conjugate in the sugar beet extracts was indole-3-acetylaspartic acid, whereas tobacco extracts contained mainly three polar IAA conjugates which were not found in the sugar beet extracts. The accumulation of the unidentified conjugates corresponded with the rise of ethylene production in the tobacco leaf discs. Reapplication of all the extracted IAA conjugates resulted in a great stimulation of ethylene production by tobacco leaf discs which was accompanied by decarboxylation of the IAA conjugates. The results suggest that in tobacco IAA-treated leaf discs the IAA conjugates could stimulate ethylene production by a slow release of free IAA. The inability of the exogenously supplied IAA to stimulate ethylene production in the sugar beet leaf discs was not due to a deficiency of free IAA within the tissue but rather to the lack of responsiveness of this tissue to IAA, probably because of an autoinhibitory mechanism existing in the sugar beet leaf discs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Patterns of ehtylene production in senescing leaves. Plant Physiol. 1979 Nov;64(5):796–800. doi: 10.1104/pp.64.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N. Relationship between Leaf Water Status and Endogenous Ethylene in Detached Leaves. Plant Physiol. 1978 Apr;61(4):658–662. doi: 10.1104/pp.61.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Effect of ethylene on the uptake, distribution, and metabolism of indoleacetic Acid-1-C and -2-C and naphthaleneacetic Acid-1-C. Plant Physiol. 1970 Jul;46(1):157–162. doi: 10.1104/pp.46.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Yang S. F. Stress-induced Ethylene Production in the Ethylene-requiring Tomato Mutant Diageotropica. Plant Physiol. 1980 Feb;65(2):327–330. doi: 10.1104/pp.65.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feung C. S., Hamilton R. H., Mumma R. O. Metabolism of Indole-3-acetic Acid: IV. Biological Properties of Amino Acid Conjugates. Plant Physiol. 1977 Jan;59(1):91–93. doi: 10.1104/pp.59.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Good N. E. Evidence That IAA Conjugates Are Slow-Release Sources of Free IAA in Plant Tissues. Plant Physiol. 1981 Dec;68(6):1424–1427. doi: 10.1104/pp.68.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Peterson M. D., Good N. E. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980 May;65(5):761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. T., Gruenert D., Knight C. A. Bound Form Indole-3-acetic Acid Synthesis in Tumorous and Nontumorous Species of Nicotiana. Plant Physiol. 1978 Jan;61(1):50–53. doi: 10.1104/pp.61.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Yang S. F. Autoinhibition of Ethylene Production in Citrus Peel Discs : SUPPRESSION OF 1-AMINOCYCLOPROPANE-1-CARBOXYLIC ACID SYNTHESIS. Plant Physiol. 1982 Mar;69(3):687–690. doi: 10.1104/pp.69.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]


