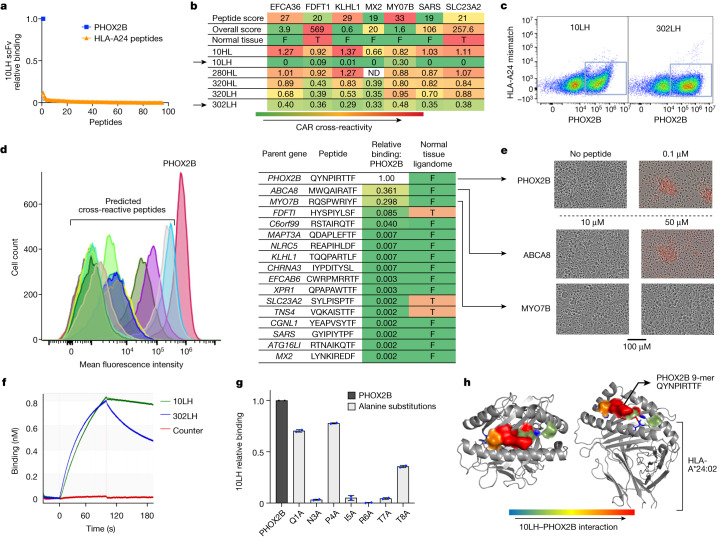
Fig. 2. Engineering pMHC-specific CARs.
a, Ranked binding affinity of 10LH scFv to PHOX2B and a panel of 95 peptides presented on HLA-A*24:02 peptides demonstrate high target binding and negligible binding to HLA-A*24:02 pMHC complexes. b, Cross-reactivity algorithm identifies CAR constructs with significant off-target binding and informs prioritization of highly selective receptors (selective receptors marked with arrows). Peptide score represents the predicted cross-reactivity based on the amino acid sequences of normal tissue peptides; overall score calculated based on peptide score, binding affinity and normal tissue expression. F, absent in normal immunopeptidome; T, peptides reported in the normal tissue immunopeptidome. c, Example counterstaining of top CAR clones with target (x axis) and off-target (y axis) peptides on HLA-A*24:02 reveals selective target binding in 10LH and 302LH constructs. d, Flow cytometry plot (left) of predicted cross-reactive peptides compared with PHOX2B shows cross-reactive binders ABCA8 (light blue) and MYO7B (gray). FDFTI is in purple and C6orf99 in dark olive green. Flow mean fluorescence intensity quantified by relative binding to PHOX2B is in table; right. e, Functional screening of ABCA8 and MYO7B shows CAR killing only through ABCA8 at a supraphysiological concentration of 50 µM compared with PHOX2B killing at 0.1 µM. ABCA8 and MYO7B were not detected in the normal tissue immunopeptidome, and none of the peptides predicted by sCRAP that were detected in the normal immunopeptidome (FDFTI, SLC23A2 and TNS4) demonstrate binding to 10LH. Experiment was performed once on entire panel of CAR constructs and repeated for 10LH and 302LH on expanded panel of peptides. f, Representative BLItz plot at 200 nM shows a fast on-rate for 10LH and 302LH and slow off-rate for 10LH (Kd = 7.6 × 10−4 s–1). g, Alanine scan of QYNPIRTTF reveals that mutations in five residues (N3A, I5A, R6A, T7A and T8A) result in significant abrogation of binding to PC-CAR 10LH (n = 2; data presented as mean). h, PHOX2B–HLA-A*24:02 crystal structure paired with alanine scan of 10LH using MHC class I tetramers allows mapping of the peptide–receptor interface, revealing spatial conformation of five receptor contact residues.