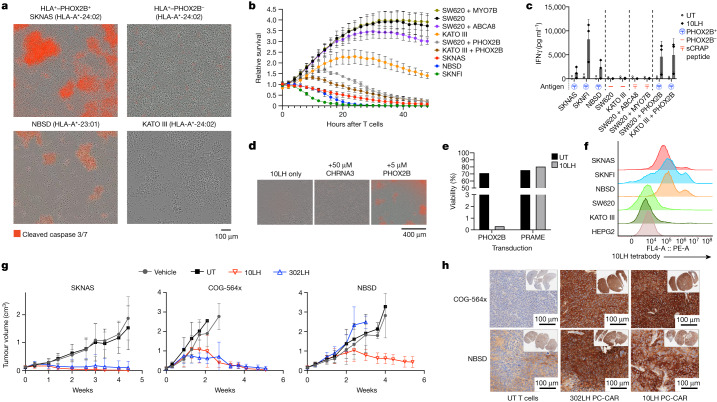
Fig. 4. PHOX2B-specific PC-CAR T cells induce potent tumour killing in vitro and in vivo and kill different HLA allotypes.
a–c, 10LH CAR induces specific killing and IFNγ release in neuroblastoma cells expressing HLA-A*24:02 and HLA-A*23:01 and PHOX2B (SKNAS, NBSD and SKNFI), but not in HLA-A*24:02–PHOX2B− non-neuroblastoma tumour cells (SW620, HEPG2 and KATO III), unless PHOX2B peptide is added. No T cell activity was observed in SW620 cells when pulsed with 10 μM of predicted cross-reactive peptides ABCA8 or MYO7B (b,c). Cytotoxicity was visualized by T cell clustering and cleaved caspase in a, relative loss of confluence measured by loss of green fluorescence in GFP-transduced cancer cells in b and IFNγ release measured by ELISA in c. Assays performed using T cells from n = 3 donors, each in triplicate; plots presented as the mean ± s.d. d, Pulsing HLA-A*24:02–PHOX2B− cell line SW620 with 5 μM PHOX2B induces complete cell killing when cultured with 10LH CAR, but no killing when pulsed with 50 μM CHRNA3. Repeated across three experiments with similar results. e, 10LH CAR specifically and specifically kills SW620 control cells transduced with PHOX2B, but not with PRAME. f, Staining cancer cells with tetramerized 10LH scFv enables the detection of PHOX2B pMHC on neuroblastoma cells but not in HLA-matched controls. g, PHOX2B-specific PC-CAR T cells induce potent tumour killing in mice engrafted with neuroblastoma PDX tumours, including the fast-growing line COG-564x and HLA-A*23:01 line NBSD. n = 6 mice enrolled per group (individual plots shown in Extended Data Fig. 17); data shown are representative from one of two independent, in vivo studies for each PDX line; shown as the mean ± s.d. h, Treatment with 10LH and 302LH PC-CARs potently upregulate HLA expression in PDX tumours collected from lone mice in each group reaching tumour burden compared with mice treated with untransduced T cells (COG-564x collected 11 days after treatment; NBSD collected 14 days after treatment for UT and 17 days after treatment for 10LH and 302LH; both tumours collected from one experiment).