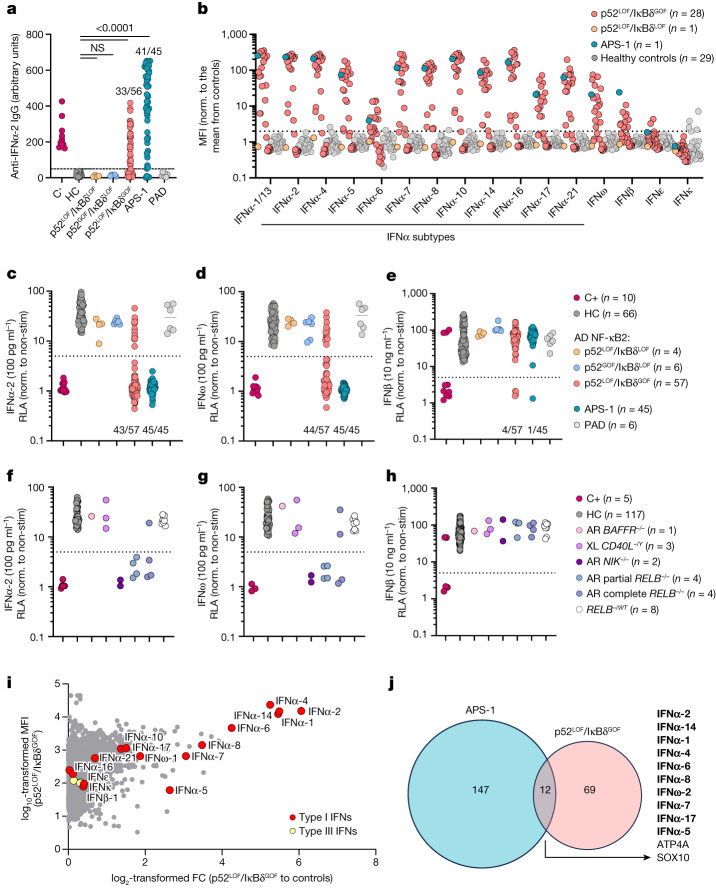
Fig. 3. AAN-I-IFNs detected in patients heterozygous for p52LOF/IκBδGOF variants and patients with inborn errors of RELB or NIK.
a, Detection of IgG autoantibodies against IFNα-2 by Gyros in patients with inborn errors of NF-κB2 with a p52LOF/IκBδLOF (n = 4), p52GOF/IκBδLOF (n = 6) or p52LOF/IκBδGOF (n = 56) variant, patients with APS-1 (n = 45), patients with idiopathic PAD (n = 6), positive control individuals with AAN-I-IFNs (C+, n = 10) or healthy control individuals (HC, n = 25). Data are the mean values from at least three independent experiments. Statistical comparisons were performed using two-tailed Mann–Whitney U-tests. NS, not significant. b, Detection, using a multiplex bead assay, of autoantibodies against the 16 type I IFNs in patients with p52LOF/IκBδGOF (n = 28) or p52LOF/IκBδLOF (n = 1) variants or with APS-1 (n = 1). Values are normalized to the mean fluorescence intensity (MFI) of plasma samples from healthy control individuals (n = 29) for each indicated cytokine. c–e, Luciferase-based neutralization assay to detect autoantibodies neutralizing 100 pg ml−1 IFNα-2 (c), IFNω (d) or 10 ng ml−1 IFNβ (e) in positive-control individuals (n = 10), healthy control individuals (n = 66), patients with a p52GOF/IκBδLOF (n = 6), p52LOF/IκBδLOF (n = 4) or p52LOF/IκBδGOF (n = 57) variant, patients with idiopathic PAD (n = 6) and patients with APS-1 (n = 45). Non-stim., non-stimulated. f–h, Luciferase-based neutralization assay to detect autoantibodies neutralizing 100 pg ml−1 IFNα-2 (f) or IFNω (g) or 10 ng ml−1 IFNβ (h) in patients with autosomal-recessive BAFFR (n = 1), X-linked (XL) CD40L deficiency (n = 3), autosomal-recessive NIK deficiency (n = 2), autosomal-recessive RELB partial or complete deficiency (n = 8) in healthy relatives heterozygous for a null or hypomorphic RELB allele (n = 8), positive control individuals (n = 5) or healthy control individuals (n = 117). All neutralization assay data are presented as the mean of at least two independent experiments. i, Protein microarray showing the distribution of autoantibody reactivity in plasma samples from patients carrying a p52LOF/IκBδGOF variant (n = 13). Data are represented as the fold change (FC) relative to 26 plasma samples from healthy control individuals. Data for HuProt are presented as the mean of at least two technical replicates. j, Representation of the global autoantigen profile of patients with APS-1 and patients with a p52LOF/IκBδGOF variant, with their overlap. Type I IFN autoantigens are highlighted in bold.