Abstract
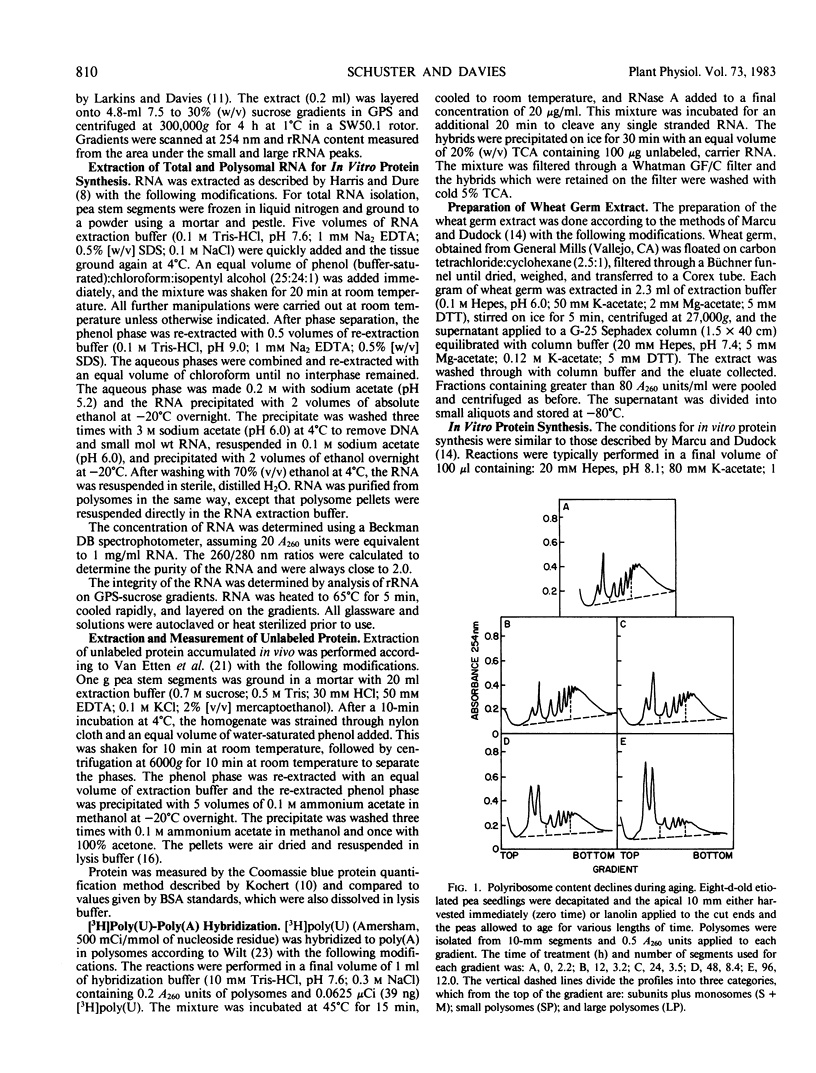
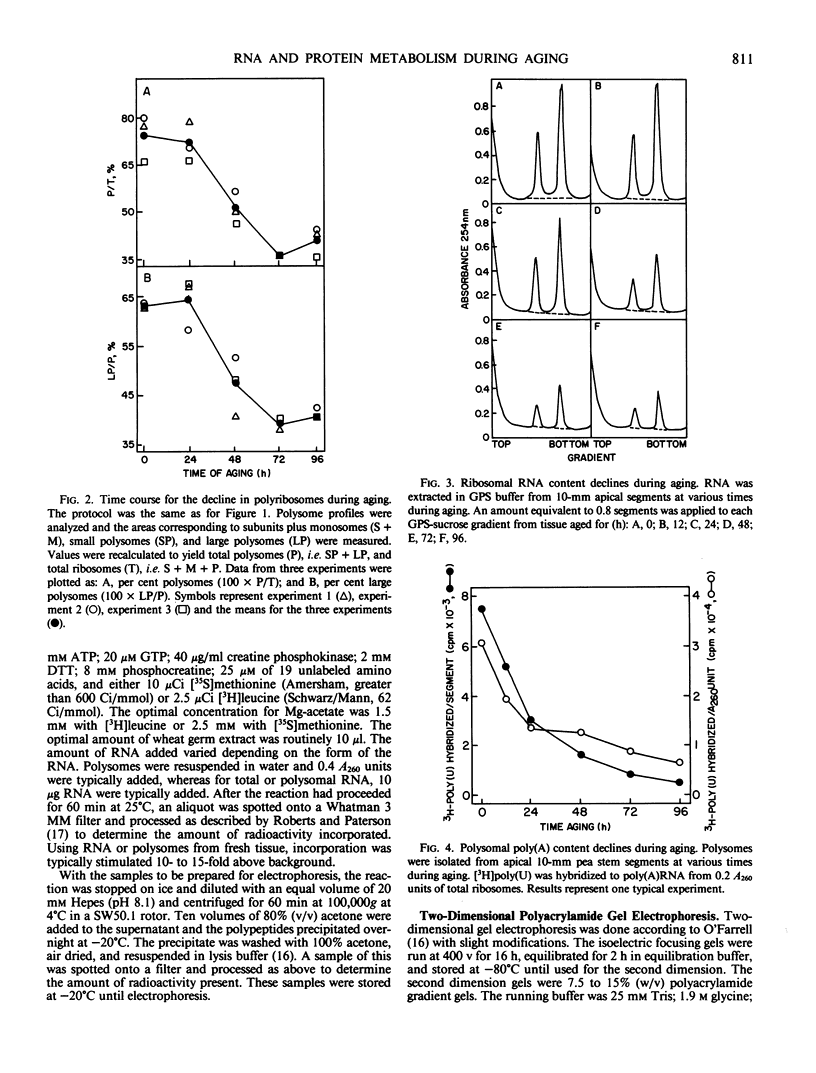
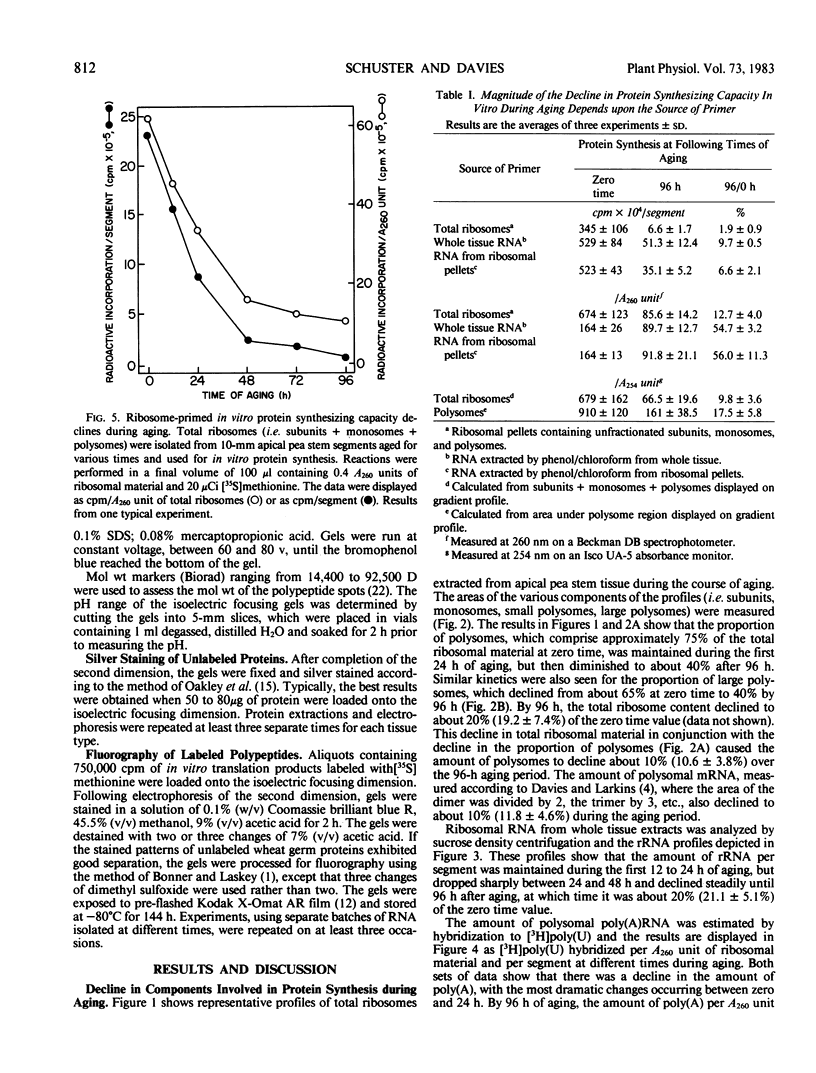
Aging of actively growing, etiolated pea Pisum sativum L. var Alaska plants was initiated by removing the plumules of plants in the third internode stage, and applying lanolin to the cut apices of otherwise intact plants. During the subsequent 4-day aging period, several degenerative events occurred in this apical 10-millimeter region. Ribosomal RNA and messenger RNA contents declined, polyribosomes disaggregated, and the protein synthesizing capacity of the polysomes decreased.
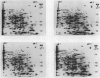
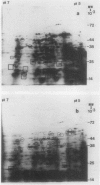
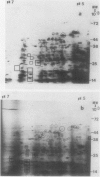
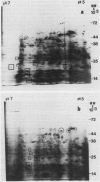
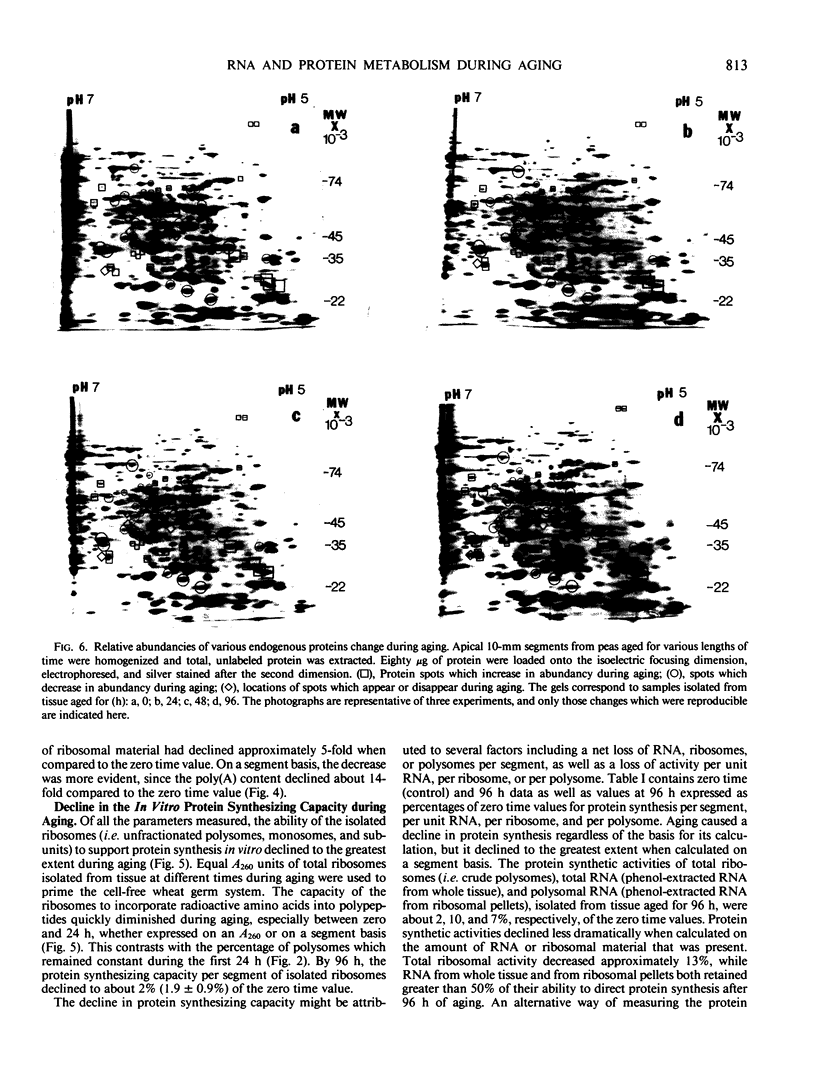
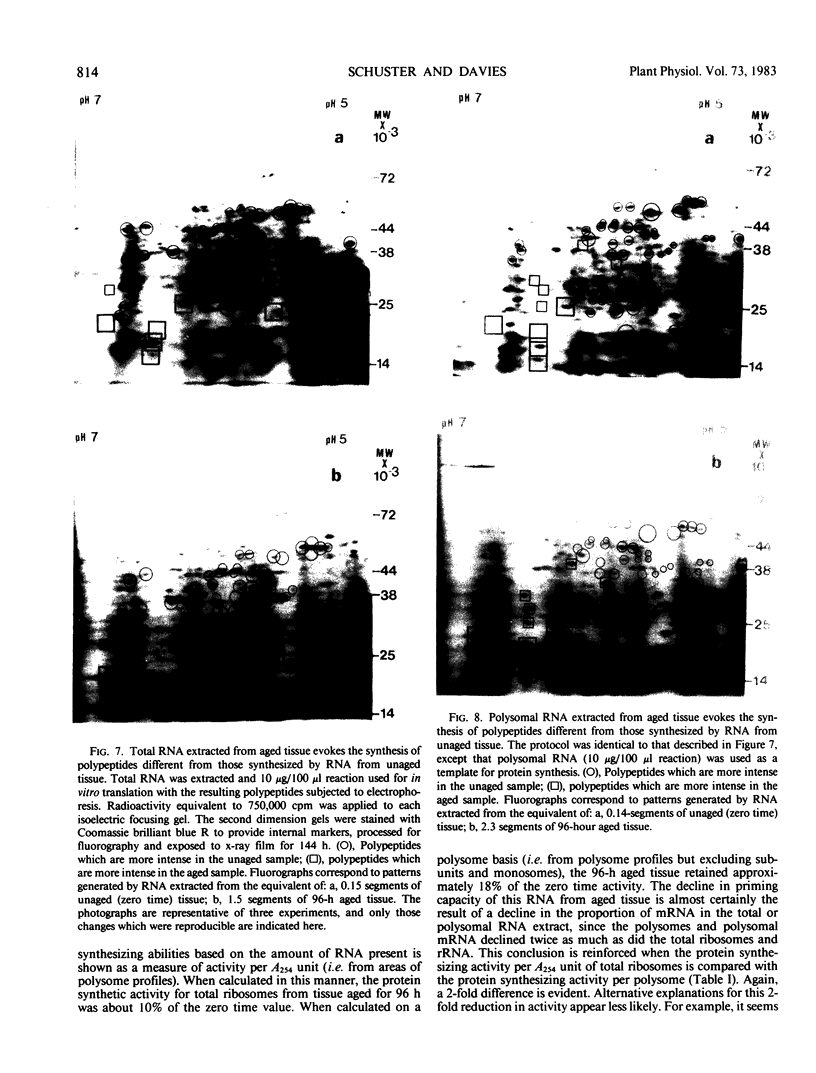
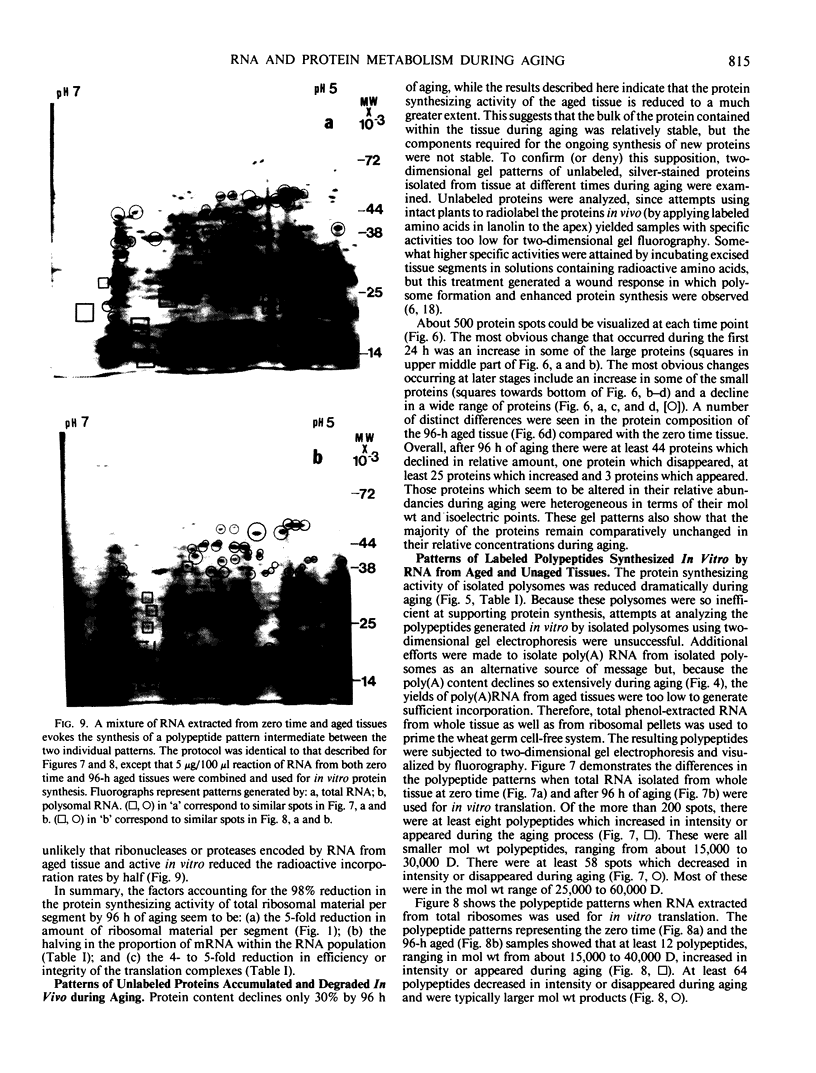
Two-dimensional, silver-stained protein patterns revealed that aging altered the relative amounts of specific cellular proteins accumulated in vivo. In addition, polypeptide patterns generated by cell-free translation of total and polysomal RNA, isolated from unaged and aged tissues, showed major modifications. More than 200 spots could be resolved by two-dimensional gel fluorography of translation products using RNA from fresh tissues. Of these 200 spots, about eight appeared or increased when total RNA from aged tissue was used, and about 58 disappeared or declined. When polysomal RNA from aged tissue was used as template, about 12 spots appeared or increased, whereas about 64 disappeared or decreased. In general, the products which increased after aging were lower molecular weight and those that decreased were higher molecular weight.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Maclachlan G. A. Indoleacetic Acid and the synthesis of glucanases and pectic enzymes. Plant Physiol. 1968 May;43(5):735–742. doi: 10.1104/pp.43.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosomes from Peas: II. Polyribosome Metabolism during Normal and Hormone-induced Growth. Plant Physiol. 1973 Oct;52(4):339–345. doi: 10.1104/pp.52.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Effects of indoleacetic acid on intracellular distribution of beta-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968 Dec;128(3):595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Davies E. Polyribosomes from Peas: VI. Auxin-stimulated Recruitment of Free Monosomes into Membrane-bound Polysomes. Plant Physiol. 1976 Apr;57(4):516–518. doi: 10.1104/pp.57.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B., Dure L., 3rd Developmental regulation in cotton seed germination: polyadenylation of stored messenger RNA. Biochemistry. 1978 Aug 8;17(16):3250–3256. doi: 10.1021/bi00609a012. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant Physiol. 1983 Nov;73(3):817–821. doi: 10.1104/pp.73.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : III. Response to Auxin in Aged Tissue. Plant Physiol. 1983 Nov;73(3):822–827. doi: 10.1104/pp.73.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Anderson J. M., Key J. L. Influence of auxin and incubation on the relative level of polyribosomes in excised soybean hypocotyl. Plant Physiol. 1973 Dec;52(6):608–612. doi: 10.1104/pp.52.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Freer S. N., McCune B. K. Presence of a major (storage?) protein in dormant spores of the fungus Botryodiplodia theobromae. J Bacteriol. 1979 May;138(2):650–652. doi: 10.1128/jb.138.2.650-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]