Abstract
Oaks (Quercus L., Fagaceae) are a widespread tree species worldwide, and in Hungary they account for nearly 30 % of the forests. Their wood is valuable, but their bark is considered as a by-product. Oak bark, available in large quantities but with no dedicated use, contains a significant amount of valuable extractives. Its (+)-catechin content is around 1 %. (+)-Catechin is mostly used for food industry, medicine and many other industrial purposes, representing a significant financial value. The aim of the present research was to compare the (+)-catechin concentrations in the bark of the most important oak species found in Hungary and to optimize sample pretreatment (conservation) and extraction methods in order to achieve fast and efficient extraction. From these species the highest concentrations were measured in Q. robur and Q. robur ssp. slavonica (8–12 mg (+)-catechin/g dry bark). The combination of microwave sample pretreatment and microwave assisted extraction proved to be the most time- and cost-effective method. The utilization of the extracted bark powder for energetic purposes requires further investigations.
Keywords: (+)-Catechin, Oak bark, Thin-layer chromatography, Microwave assisted extraction, Enzyme inactivation
1. Introduction
Wastes from the food, forestry, and agricultural industry make promising raw materials not only because they are inexpensive but also because their re-use benefits the environment [1]. The bark of forest trees is particularly important in this regard [[2], [3], [4], [5]]: according to the data of the Food and Agriculture Organization of the United Nations, worldwide roundwood production was 3968 M m3 in 2021 [6] which generated approximately 397 M m3 bark waste by assuming an average bark ratio of 10 %. Values for Hungary are summarized in Table 1, broken down to individual tree species. According to Tables 1 and in Hungary the Quercus spp. occupy the most area, and have the highest logging volume, therefore the volume of generated bark waste, too.
Table 1.
The proportion of woody species by occupied area [10], wood logging volume [11] and estimated bark volume in Hungary based on the data of 2021.
| Species | Occupied area (1000 ha) | Wood logging (1000 m3) | Estimated bark volume (m3) |
|---|---|---|---|
| Quercus petraea and robur L. | 391 | 972 | 97,200 |
| Quercus cerris L. | 216 | 801 | 80,001 |
| Fagus sylvatica L. | 114 | 683 | 68,300 |
| Carpinus betulus L. | 97 | 234 | 23,400 |
| Robinia pseudoacacia L. | 459 | 1421 | 142,100 |
| Other high density hardwood species | 124 | 341 | 34,100 |
| Hybrid poplar | 198a | 1306 | 130,600 |
| Indigenous poplar | 287 | 28,700 | |
| Salix spp. | n/a | 39 | 39,000 |
| Other low density hardwood species | 96 | 241 | 24,100 |
| Pinus sylvestris L. | 106 | n/a | n/a |
| Pinus nigra J.F.Arnold | 58 | 1198b | 119,800 |
| Other conifers | 15 | n/a | n/a |
| Total | 1876 | 7523 | 752,300 |
sum of poplars (Populus spp.).
sum of all coniferous species.
Oaks (Quercus L., Fagaceae) are woody angiosperms, that comprise approximately 400–500 species [7], mostly found in Central America and Southeast Asia, while fewer species occur in Western North America, Eurasia, and in the Mediterranean region [[7], [8], [9]]. In Hungary seven species are common, out of which four are considered native to the country: sessile oak (Quercus petraea Liebl.), pedunculate oak (Quercus robur L.), downy oak (Quercus pubescens Willd.), and Turkey oak (Quercus cerris L.). Though the number of oak species in Hungary is low, they occupy over 30 % of the country's total forest area (Table 1), providing their outstanding domestic ecological and economic significance.
The bark of oak species contains a significant amount (6–20 %) of extractable substances, primarily phenolic acids (gallic acid, ellagic acid), condensed tannins (e.g. procyanidin B dimer), hydrolysable tannins (isomers of monogalloyl to pentagalloyl glucoses), flavonoids (taxifolin and quercetin glycosides, (+)-catechin, catechin-gallate) [12,13]. In the past, the most typical chemical utilization of oak bark was the extraction of tannins and phytoactive substances. The latest studies found in scientific literature investigated the extractive composition [[14], [15], [16], [17]] as well as the biological activity [18] and wound-healing properties [19] of the bark of oak species.
(+)-Catechin (Fig. 1) belongs to the group of flavan-3-ol compounds (generally referred to as catechins) and is one of the identified polyphenols in the bark of oaks, with numerous beneficial physiological (antioxidant, radical scavenging-, anti-inflammatory-, antibacterial, antitumor) effects as well as iron chelating properties [20,21]. Catechins are naturally present in bark, wine, tea, fruits and chocolate and can have a peroxyl radical scavenging activity ten times higher than L-ascorbate and β-carotene when tested on bacteria [22]. In recent years, catechins have been used as natural antioxidants in oils and fats against lipid oxidation, supplement for animal feeds both to improve animal health and to protect animal products, as antimicrobial agents in foodstuffs and as a health functional ingredient in various foods and dietary supplements [23]. Strong antioxidant effects of catechins is expected to produce many advances in the food, cosmetics, and pharmaceutical industries [24]. Particularly, (+)-catechin is used among others, in medicine, in clinical trials [[25], [26], [27]] and in the filter system of air conditioning equipment [28].
Fig. 1.

The structure of (+)-catechin.
The research, development and application activities related to (+)-catechin require the production of (+)-catechin with sufficient amount and purity. This is primarily done by isolation and purification from plant tissues (e.g. green tea, peppery bitterwort (Polygonum hydropiper L.), Java plum (Syzygium cumini (L.) Skeels), etc.) [23,29,30].
There have been earlier studies on the optimization of the extraction of (+)-catechin using various methods. Bhadange et al. proved that ultrasonication enhances catechin yield and it is also energy efficient. However longer exposures may reduce the yields due to the degradation of catechin into smaller molecules, thus ultrasonication should be used in a controlled manner [30]. Besides ultrasonication, microwave-assisted extraction (MAE) has also been used extensively for the extraction of (+)-catechin from various plant materials (e.g. tea, grape seeds) [[31], [32], [33]]. Researchers concluded that optimization is also essential with this method, as flavan-3-ols, and particularly catechin, are susceptible to degradation under various conditions during MAE [34].
Not only extraction methods, but also storage of plant material have effects on (+)-catechin content. According to Dedrie et al. the debarking process and the storage conditions after debarking have a detrimental effect on the chemical composition (particularly on the content of (+) catechin and other extractives) of oak bark due to degradation effects [12]. The same was concluded for the bark of other woody species [13,35,36]. These results outline the importance of the optimization of storage and pretreatment conditions of bark for achieving highest yields of (+)-catechin.
The present study focused on the extraction of (+)-catechin from the bark of oak species growing in Hungary. Beside the national significance of oak species, the choice of basic material is also justified by the fact that the extracted bark material is more suitable for the biogas production via anaerobic fermentation compared to unextracted bark, due to its lower ash and extractive content [37]. The (+)-catechin content of the bark of different oak species was compared and pretreatment methods were tested aiming the preservation of the (+)-catechin content. Intensification of the extraction process was carried out by comparing different extraction methods as well as by the elaboration of a preparative purification method for the fast and low-cost extraction of (+)-catechin from oak bark. Results could also contribute to the complex biorefinery valorization of extracted oak bark for other purposes (e.g. biogas production).
2. Materials and methods
2.1. Sample collection and preparation
Samples originated from the Szárhalmi forest in Sopron Mountains (H) from forest compartments Sopron 56A, 52A and 52 B. The logs were 38 years (Sopron 56A) and 23 years (Sopron 52 A and B). Investigated taxa were as follows: red oak (Quercus rubra L.), Turkey oak (Quercus cerris L.), pedunculate oak (Quercus robur L. and Quercus robur ssp. slavonica (Gáyer) Mátyás) and sessile oak aggregate (Quercus petraea (Matt). Liebl, Quercus dalechampii Ten. and Quercus polycarpa (Schur) Soó). In the case of pedunculate oak, one subspecies (subsp. slavonica) and in the case of sessile oak aggregate 3 different microspecies (petraea, dalechampii and polycarpa) were identified by the morphology of the leaves and bark. One healthy tree was investigated from each taxa. After felling, the trees were debarked with an axe up to the height of 1.5 m. Altogether 800–1000 g of fresh bark were collected from each log. Collected bark samples comprised of large (10–20 cm long) pieces, further chopping was avoided. Samples were taken to the laboratory and processed immeadiately.
2.2. Enzyme inactivation
Samples were divided into four equal fractions (around 250 g each). Fractions were treated and labeled as follows: c: control, 250 g bark with no enzyme inactivation treatment, ground immediately; m: 250 g bark treated in a household microwave oven (Vision MMO700) at 700 W for 3 min, u: 250 g bark treated with UV irradiation (λ = 254 nm) for 10 min using a 20 W UV-light source; h: 250 g bark heated at 105–110 °C for 15 min in a drying chamber. After treatment fractions were chopped into small (1 cm) pieces, ground in a hammer mill and sieved. The sieve fraction <1 mm was used for further investigations.
2.3. Determination of enzyme activity
About 0.6 g sample was homogenized vigorously with 15 mL phosphate puffer (pH: 6.0) for 10 min then centrifuged at 6000 min−1 for 10 min. The supernatant was collected and taken to analysis. The peroxidase enzyme (POD) activity was determined using the method of Shannon et al. measuring absorbance change at 480 nm and regarding 0.01 ΔA min−1 as 1 Unit [38]. The polyphenol-oxidase enzyme (PPO) activity was assayed according to the method of Flurkey and Jen at 420 nm, taking 0.001 ΔA min−1 as 1 Unit [39]. All measurements were conducted in triplicates.
2.4. Extraction of (+)-catechin
In all experiments ethanol:water 80:20 v/v was used as the solvent. After all extractions, the solutions were filtered using Whatman GF/A glass fiber syringe filter and taken to analysis. All extractions were conducted in triplicates. For stirring extraction, a Variomag Poly 15 magnetic stirrer was applied, for MAE a Vision MMO700 type household microwave oven was used, and Soxhlet extraction was done in a conventional Soxhlet apparatus.
For bark preservation optimization the following extraction protocol was applied: 0.1 g sample was homogenized with 25 mL solvent for 3 h using a magnetic stirrer.
For extraction intensification analyses microwave-inactivated Q. robur and Q. robur ssp. slavonica samples were used and following extraction protocols were applied: (1) three different methods were compared for extraction dynamics: (A) stirring of 0.1 g sample with 25 mL solvent for 1, 4, 6 and 12 h, (B) Soxhlet extraction of 0.4 g sample with 100 mL solvent for 1, 4, 6 and 12 h, (C) MAE of 0.1 g sample with 25 mL solvent using 2, 5, 8, 12 and 20 extraction cycles (1 cycle: 15 s microwave treatment of the mixture with 700 W irradiation followed by 45 s cooling in a cold water bath) and adjusting end volume to 25 mL with neat solvent.
(2) for large-scale extraction 3 g sample was extracted with 100 mL solvent by stirring, Soxhlet extraction and MAE using previously-determined optimum time settings as follows: (A) 3 step extraction by stirring for 3 × 2 h, using 40 mL + 30 mL + 30 mL amounts of solvent and combining extracts to an end volume of 100 mL, (B) Soxhlet extraction for 4 h and adjusting end volume to 100 mL, (C) 3 step MAE extraction using 40 mL + 30 mL + 30 mL amounts of solvent, each step consisted of 5 cycles (1 cycle: 10 s microwave treatment of the mixture with 700 W irradiation followed by 40 s cooling in a cold water bath) and adjusting end volume to 100 mL (total extraction time: 12.5 min).
2.5. Preparative isolation of (+)-catechin
Microwave-inactivated Q. robur ssp. slavonica bark (45 g) was extracted by applying the 3 step MAE large-scale extraction protocol described in the previous section using 1500 mL 80:20 ethanol:water v/v solution (600 mL + 450 mL + 450 mL) and applying 150 s irradiation and 600 s cooling cycles. The ethanol content was removed by rotary evaporation (40 °C) and (+)-catechin was extracted from the remaining aqueous phase using 2 × 500 mL ethyl-acetate. The ethyl-acetate fractions were combined and evaporated to dryness using rotary evaporation at 40 °C (Rotavapor, Büchi, Flavil, Switzerland). The remaining dry solids were redissolved with 5 mL ethanol and taken to analysis. Preparative isolation of (+)-catechin was carried out based on the method of Jerez et al. using Sephadex LH-20 gel stationary phase filled in a 1 cm × 8 cm glass column. 0.3 mL of the concentrated extract was introduced at the top of the column, and eluted with 20 mL solutions of methanol:water 60:40 v/v, methanol:water 75:15 v/v and methanol:water 90:10 v/v, collecting 2 mL fractions (altogether 30) [40].
2.6. High-performance thin layer chromatographic determination of (+)-catechin content
15 mL of the extracts were evaporated to dryness under reduced pressure, using rotary evaporation at 40 °C (Rotavapor, Büchi, Flavil, Switzerland). Dry solids were redissolved in 1 mL ethanol and taken to chromatographic analysis. The quantitative determination of (+)-catechin was done using high-performance thin layer chromatography (HPTLC) followed by derivatization with vanillin-sulfuric acid reagent and subsequent densitometric evaluation basing on a procedure detailed in Hofmann et al. [41].
2.7. Statistical analyses
For the comparison of (+)-catechin concentrations one-way analysis of variance (ANOVA) was run using Statistica software (Version: 12; StatSoft Inc., Tulsa, OK, USA) software and the Tukey HSD calculation method was applied as a post-hoc test at p < 0.05 level. In order to fulfil the requirements of the ANOVA analysis, values of the measurements were first checked for normal distribution, and then the variables were checked for the homogeneity of variances using Bartlett's Chi-square test.
2.8. Chemicals
Double distilled water used for the extractions was prepared using conventional distillation equipment. Ethanol, methanol, ethyl-acetate (analytical grade), formic acid and sulfuric acid were obtained from VWR International (Budapest, Hungary). Catechol, potassium hydrogen phosphate, potassium dihydrogen phosphate and hydrogen peroxide were purchased from Reanal (Budapest). Diisopropyl ether, (+)-catechin, o-dianisidine and vanillin were obtained from Honeywell (aka. Fluka).
3. Results and discussion
3.1. Bark preservation optimization
3.1.1. Enzyme inactivation
During our preliminary experiments, we observed that the peeled bark quickly turns brown and (+)-catechin content decreased rapidly presumably as a result of the oxidation processes. These tendecies can be seen well in Fig. 2. The initial high (+)-catechin content decreased rapidly. On the fifth day only half of the original concentration remained. The highest values were found in Q. robur and Q. robur ssp. slavonica (7–9 mg (+)-catechin/g dry bark). Interestingly the content of (+)-catechin in the bark of different microspecies of sessile oak aggregate was significantly lower compared to that of the pedunculate oaks, despite the fact that sessile and pedunculate oaks are closely related, with a tendecy of cross-pollination [7]. Exceptionally low values were found for Quercus polycarpa even in repeated experiments, which requires further explanation.
Fig. 2.
Change of the (+)-catechin content in untreated bark samples during storage.
Results are in accordance with the data of Dedrie et al. who outlined that storage conditions after debarking reduced the percentage of (+)-catechin and other phenolic extractives, presumably due to the degradation effects [12]. Oxidoreductase enzymes (primarily peroxidase and polyphenol oxidase) are responsible for the rapid oxidation of (+)-catechin [42,43]. Thus to achieve preservation of (+)-catechin content, first these enzymes must be inactivated: preservation procedures had to be developed and implemented, for efficient preservation the (+)-catechin content of the processed and stored bark.
Three methods were selected from the literature and compared, which are applied at laboratory and industrial scale for the inactivation of oxidizing enzymes for the preservation of foodstuffs: microwave treatment [44], treatment with UV irradiation [45] and thermal treatment [46]. Inactivation methods were applied on freshly peeled bark pieces as described in chapter 2.2. After the treatments, samples were ground, which turned out to be more favorable compared to the grinding of fresh bark, since the bark had lost the majority of its moisture content during the treatments, so the grinding process became easier.
The efficiency of enzyme inactivation procedures was assessed by the determination of the remaining POD and PPO enzyme activities of the samples and comparing to untreated samples’ values.
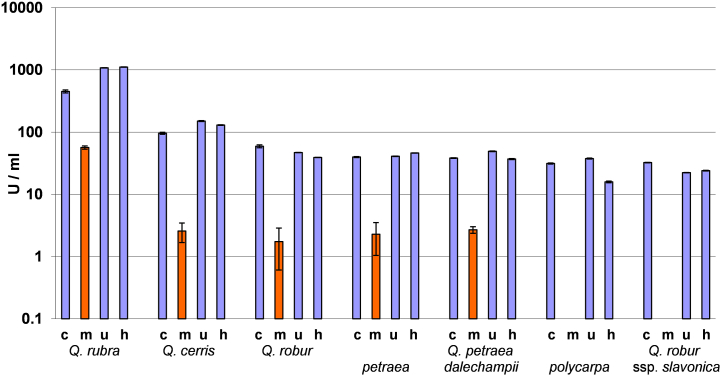
Fig. 3, Fig. 4 summarize the results of these three inactivation methods compared to untreated samples. It was concluded that the enzyme activity in the UV- and heat-treated samples remained unchanged or was only slightly reduced compared to the untreated sample. While applying the microwave treatment a very high (90–99 %) decrease of the POD and PPO activity was achieved. According to the results the microwave treatment was best suited from the investigated methods for the inactivation of the tested enzymes in oak bark.
Fig. 3.
The activity of polyphenol oxidase (PPO) enzyme in untreated (c) and inactivated (m: microwave-treated, u: UV-treated, h: heat-treated) bark samples. Error bars: standard deviation.
Fig. 4.
The activity of peroxidase (POD) enzyme in untreated (c) and inactivated (m: microwave-treated, u: UV-treated, h: heat-treated) bark samples. Error bars: standard deviation.
Despite the fact that UV radiation is widely used for food preservation purposes and for the reduction of browning (e.g. in apple juice), due to its inactivating effect on oxidase enzymes [45,47] it proved to be effective only to a limited extent on the present bark samples. A possible explanation for this, is that UV radiation is only absorbed in the surface layers and can only exert its effect there, not deep in the tissues. Thus majority of the enzymes remain unaffected by the treatment. According to Vamos-Vigyazo and Haard the exposure of PPO to temperatures of 70–90 °C destroys their catalytic activity [48]. Although the food industrial use of thermal enzyme inactivation has been researched and found efficient [46], in the present study the treatment of the samples for 15 min at 105–110 °C resulted no or only slight loss in the activity of POD and PPO enzymes. This could possibly be explained by the high moisture content of fresh bark (30–40 %, data not shown) which delayed the rise of the temperature within the bark samples, resulting low enzyme inactivation efficiency.
3.1.2. The time course of (+)-catechin concentration in conserved samples
In order to compare the (+)-catechin-preserving effects of the inactivation procedures, samples (including control) were extracted and their (+)-catechin content was determined at days 0, 7, 28, and 56 after the treatments. In these measurements, only those species were tested which showed the overall highest levels of (+)-catechin (Q. robur and Q. robur ssp. slavonica). The time course of the (+)-catechin contents in the enzyme-inactivated and control samples is depicted in Fig. 5A (Q. robur) and Fig. 5B (Q. robur ssp. slavonica).
Fig. 5.
The time course of the (+)-catechin concentrations in Quercus robur (A) and Quercus robur ssp. slavonica (B) bark samples using different enzyme inactivation techniques. Error bars: standard deviation.
Based on Fig. 5, it was verified again that the most effective preservation method for the tested samples was the microwave treatment, as these samples retained best their initial high concentration of (+)-catechin and showed the lowest decrease over time. The enzyme-inactivating and polyphenol-preserving effects of the microwave treatment on the tissues of forest trees have already been confirmed by the authors' previous works [35,36,49]: they explained the efficiency of the treatment by the rapid heating affecting both the surface and inner parts of the sample and by the rapid loss of moisture.
Using thermal and UV inactivation, more (+)-catechin was retained than in the control sample, but significantly lower values were measured compared to microwave treatment, which is in accordance with the enzyme inactivation results presented in the previous chapter. The worst overall results of thermal inactivation was explained by the fact that due its high moisture content, the slow warming of the sample temporarily increased enzyme activities, contributing to the degradation of (+)-catechin.
3.2. Extraction intensification
3.2.1. Comparison of different extraction methods
After choosing the best sample preservation method, we focused on the comparison of extraction methods capable of extracting the largest amounts of (+)-catechin from the bark in the fastest and most efficient way. Based on literature data [40,50,51] we selected three extraction methods: Soxhlet, continuous stirring and MAE, and compared them in terms of time and cost. In each procedure, 0.1 g of bark sample was treated with 25 mL extraction solvent, representing a solid (g):liquid (mL) ratio of 1:250. For the selection of the time of extraction, we also recorded dissolution curves, by which we determined how long it was necessary to extract using a given method to reach quasi-equilibrium extraction with the given solid:liquid ratio. Table 2 summarizes the results of the experiments.
Table 2.
Comparison of different extraction methods using a soild (g):liquid (mL) ration of 1:250. Results are indicated as average ± standard deviation. Different letters in a column (species) indicate significant differences as p < 0.05 level.
| Q. robur | Q. robur ssp. slavonica | ||
|---|---|---|---|
| Method |
Time (min) |
mg (+)-catechin/g dry bark |
|
| Stirring |
60 | 4.68 ± 0.21a | 4.84 ± 0.49 ABCD |
| 240 | 7.63 ± 0.51c | 6.48 ± 0.21 E | |
| 360 | 7.82 ± 0.37c | 7.73 ± 0.28 F | |
| 720 |
7.00 ± 0.60 bc |
7.34 ± 0.22 F |
|
| Soxhlet |
60 | 6.23 ± 0.75 b | 3.78 ± 0.47A |
| 240 | 7.45 ± 0.66c | 4.95 ± 0.33 BD | |
| 360 | 7.18 ± 0.28 bc | 4.69 ± 0.16 AB | |
| 720 |
7.13 ± 0.62 bc |
4.87 ± 0.37 BCD |
|
| Microwave | 2 | 6.52 ± 0.64 b | 5.43 ± 0.22 C |
| 5 | 5.82 ± 0.49 b | 4.81 ± 0.17 B | |
| 8 | 7.63 ± 0.07c | 5.38 ± 0.10 CD | |
| 12 | 7.36 ± 0.16 bc | 5.21 ± 0.22 BCD | |
| 20 | 4.74 ± 0.40a | 5.45 ± 0.07 BCD | |
According to the data in Table 2 the highest concentrations were measured between 4 and 6 h for continuous stirring extraction, and after 4 h in the Soxhlet process, for both wood species. By applying longer extraction times no significant increase could be reached.
Using MAE, the maximum concentrations were measured between 8 and 12 min. The high value of Q. robur ssp. slavonica at 2 min is presumably the result of a measurement error, which was not interpreted. In the case of Q. robur, after 20 min of extraction, there was a significant decrease of the (+)-catechin concentration, presumably due to degradation effects.
The results are in accordance with the findings of Bouras et al. who demonstrated the efficiency of MAE in terms of speed and extracted concentrations compared to other extraction methods (heat reflux, solvent extraction at 25 °C) for the extraction of oak bark polyphenols. The authors also outlined the potential degradation effects of MAE technique [52].
3.2.2. Large scale extraction experiments
The comparison of the extraction procedures was also carried out using larger amounts of sample and solvent and elevated solid:liquid ratio (1:33.3), which is more comparable to industrial applications. We determined and compared the extraction efficiency, the yield and the costs of the extraction of the investigated methods. In order to further increase extraction yield, we performed the stirring and MAE extraction in 3 consecutive steps, instead of the single-step continuous procedure, and in the case of the MAE we also changed the cycle time. For the Soxhlet extraction, the protocol remained the same. We applied the extraction times defined in the previous chapter as a basis: 12.5 min for MAE, 4 h in the case of Soxhlet extraction, and 6 h in the case of stirring.
Fig. 6A shows the concentrations obtained in the large-scale extraction experiment for the two tested species. Higher values were obtained compared to the values presented in the previous chapter. This was especially true for Q. robur ssp. slavonica, which can be explained by the higher solid:liquid ratio and in the case of the stirring and MAE processes, by the higher extraction efficiency of the 3 step process compared to the single step process. Within a species there was no significant difference between the concentrations achieved by the different extraction techniques. The bark of Q. robur ssp. slavonica contained a significantly higher level of (+)-catechin (10.8–13.0 mg (+)-catechin/g dry bark) compared to that of Q. robur (7.21–8.04 mg (+)-catechin/g dry bark), which is accordance with the findings of the trends shown in Fig. 2, Fig. 5 Comparing to other types of samples, it was found that measured values were lower than the (+)-catechin concentration determined for green tea by MAE technique (15–37 mg/g) [32] and for cultured cells of Polygonum hydropiper 4–29 mg/g [29].
Fig. 6.
The (+)-catechin content of the samples using the large-scale extraction methods and the applied extraction times. Error bars represent standard deviation, different letters denote significant differences at p < 0.05 level (A). Extraction yield of the different methods in mg (+)-catechin/(g dry bark · hour) (B). S: stirring extraction, M:MAE, X: Soxhlet extraction.
By relating the results on the time needed for extraction, we get the amount that can be extracted from 1 g of dry bark in an hour with the given method (yield) in mg (+)-catechin/(g dry bark · hour) unit (Fig. 6B). Overall, MAE proved to be significantly faster and about 15–30 times more efficient compared to the other two methods. With the presented 3-step MAE method 36.89 (+)-catechin/(g dry bark · hour) yield was reached for Q. robur and 51.55 mg (+)-catechin/(g dry bark · hour) for Q. robur ssp. slavonica.
Several studies have already proven the efficiency of MAE for the extraction of (+)-catechin from plant samples [[31], [32], [33]]. However these experiments were carried out for analytical purposes which require special equipment and use small amounts (0.5–1 g) of plant material [33,53,54]. The advantage of the presented MAE process is that it does not require a special equipment and it can also be implemented for larger quantities of samples.
Table 3 indicates the total cost associated with extraction, taking into account the time and cost implications of each method. To achieve the same (+)-catechin concentration, the three-step MAE method was not only the fastest, but also the most cost-effective: compared to the other 2 methods; its total cost is 50x lower. Stirring requires high amounts of electric power, while Soxhlet extraction, in addition to the electrical power required, also has considerable water consumption, which makes it the least cost-effective process.
Table 3.
Net costs of extraction procedures. Duration of MAE was 12.5 min (the total time of power uptake was 3 · 5 · 10 s = 150 s = 0.042 h, where 3 is the number of extraction steps, 5 is the number of extraction cycles per step and 10 s in the microwave device on-time in a cycle. Electric power was calculated using formula W U·I·t. The cost of 1 kWh electric current was 0.117 Euro, the cost of 1 m3 water was 1.32 Euro (prices for Hungary as of June 2023, calculated in Euros).
| U (V) | I (A) | Extraction time (h) | Water consumption (l/h) | Electric power (Wh) | Water costs (Euro) | Electric power costs (Euro) | Total costs (Euro) | |
|---|---|---|---|---|---|---|---|---|
| Stirring | 220 | 2 | 6 | – | 2640 | – | 0.310 | 0.310 |
| Microwave | 220 | 5.8 | 0.042 | – | 53.16 | – | 0.006 | 0.006 |
| Soxhlet | 220 | 0.85 | 4 | 40 | 748 | 0.211 | 0.088 | 0.299 |
3.3. Preparative isolation of (+)-catechin
After optimizing the sample preparation and extraction methods, our goal was to develop a method suitable for the isolation of (+)-catechin in large quantities and high purity from oak bark. The method of Jerez et al. developed for the isolation and purification of catechins from the bark of Pinus species using preparative chromatography with Sephadex LH20 stationary phase was adapted for this purpose [40]. In this experiment only the microwave inactivated bark of Q. robur ssp. slavonica was investigated, being the species with the highest overall level of (+)-catechin in this research.
During the preparative separation, a total of thirty fractions were collected per 2 mL. The composition of the fractions was examined by thin-layer chromatographic separation, using visualization with vanillin-sulfuric acid reagent, which forms a red color with catechin-type compounds. According to Fig. 7 (+)-catechin was found in the largest amount in fractions 10–13, with the highest concentration in fraction 11.
Fig. 7.
Checking the composition of fractions using thin-layer chromatography. C: (+)-catechin, EC: (−)-epicatechin. Visualization: vanillin-sulfuric acid reagent. Original version of the figure is found in Supplementary material (Fig. S1).
The result of the preparative separation is a purified fraction, the purity of which characterizes the efficiency of the separation. Therefore, we investigated the purity of fraction 11, which contains the highest concentration of (+)-catechin. Since the vanillin-sulfuric acid visualization method does not form colored derivatives with many compounds, the chromatogram in Fig. 7 does not show all possible contaminants. In order to check the purity, the same layer obtained after the chromatographic separation, but before the vanillin-sulfuric acid development, was evaluated densitometrically, thereby estimating the purity of the fraction. The result is illustrated in Fig. 8A (densitogram of the fractions) and in Fig. 8B (purity check of the fractions).
Fig. 8.
Investigation of the composition of fraction 11 using densitometric evaluation (prior to the visualization using vanillin-sulfuric acid reagent) at 280 nm in absoprtion mode. The densitogram of the whole plate (fractions 1–30) (A) and the densitogram of fraction 11 (B). C: (+)-catechin, EC: (−)-epicatechin.
By integrating the densitogram of fraction 11, we assessed that the peak area of (+)-catechin is about 60 % of the sum of the areas under all chromatographic peaks, which corresponds to the overall purity of (+)-catechin in this fraction. This can be further improved by optimizing the conditions of the preparative separation. The results are comparable to the finiding of Gong et al. who was able to purify (>90 %) catechin compounds from tea leaf extract in two rounds using semi-preparative HPLC [55].
4. Conclusions
Oaks make up a significant part of Hungary's forests, their bark provides a significant amount of raw material, and it also contains a high concentration of extractable substances. The aim of our study was to compare the (+)-catechin content of the bark of selected oak species, and to develop procedures that are suitable for the time- and cost-effective extraction and purification of the compound using process optimization and intensification approach. We developed and compared methods for preserving oak bark samples, of which microwave pretreatment proved to be the most efficient. Comparing the oak species, we found that the highest concentrations were detected in the bark of Q. robur and Q. robur ssp. slavonica: 8–12 mg (+)-catechin/g dry bark (0.8–1.2 % by weight). Q. polycarpa showed exceptionally low levels of (+)-catechin which requires further investigations. During the extraction intensification experiments, it was found that microwave-assisted extraction produced 15-30-times higher yield (36.89–51.55 mg (+)-catechin/(g dry bark · hour)) compared to Soxhlet (2.01–3.26 mg (+)-catechin/(g dry bark · hour)) and stirring extraction (1.20–1.79 mg (+)-catechin/(g dry bark · hour)). The combination of the microwave treatment for enzyme inactivation and the microwave-assisted extraction was found to be the most time- and cost-effective among the investigated procedures. To the best of our knowledge the present study is the first to apply microwave treatment for the enzyme inactivation of oak bark to preserve (+)-catechin content and for the aimed extraction of (+)-catechin from oak bark. As opposed to traditional lab-scale procedures the presented methods can also be upscaled for the processing of large amounts of bark using the applied equipment. Based on the literature, a method was adapted for the for the preparative separation and purification of (+)-catechin, which resulted in (+)-catechin of approximately 60 % purity from the bark of Q. robur ssp. slavonica. Future optimization of the purification needs to be carried out to improve (+)-catechin purity, which promotes the applicability of (+)-catechin from oak bark. The utilization of the ground bark remaining after processing and extraction, mainly for heat energy and biogas production should also be investigated in the future.
Data availability statement
Data will be made available on request.
Funding statement
Project no. FK 142527 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the “FK22 OTKA” funding scheme.
CRediT authorship contribution statement
Tamás Hofmann: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Ádám Nándor Makk: Writing – original draft, Investigation, Formal analysis, Data curation. Levente Albert: Writing – review & editing, Writing – original draft, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:T. Hofmann reports financial support was provided by National, Research, Development and Innovation Office. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22024.
Contributor Information
Tamás Hofmann, Email: hofmann.tamas@uni-sopron.hu.
Ádám Nándor Makk, Email: makkadamnandor@gmail.com.
Levente Albert, Email: albert.levente@uni-sopron.hu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vázquez G., Santos J., Freire M.S., Antorrena G., González-Álvarez J. Extraction of antioxidants from eucalyptus (Eucalyptus globulus) bark. Wood Sci. Technol. 2012;46:443–457. doi: 10.1007/s00226-011-0418-y. [DOI] [Google Scholar]
- 2.Pietarinen S.P., Willför S.M., Ahotupa M.O., Hemming J.E., Holmbom B.R. Knotwood and bark extracts: strong antioxidants from waste materials. J. Wood Sci. 2006;52:436–444. doi: 10.1007/s10086-005-0780-1. [DOI] [Google Scholar]
- 3.Diouf P.N., Stevanovic T., Cloutier A. Study on chemical composition, antioxidant and anti-inflammatory activities of hot water extract from Picea mariana bark and its proanthocyanidin-rich fractions. Food Chem. 2009;113:897–902. doi: 10.1016/j.foodchem.2008.08.016. [DOI] [Google Scholar]
- 4.Ekman A., Campos M., Lindahl S., Co M., Börjesson P., Karlsson E.N., Turner C. Bioresource utilisation by sustainable technologies in new value-added biorefinery concepts – two case studies from food and forest industry. J. Clean. Prod. 2013;57:46–58. doi: 10.1016/j.jclepro.2013.06.003. [DOI] [Google Scholar]
- 5.Ghitescu R.-E., Volf I., Carausu C., Bühlmann A.-M., Gilca I.A., Popa V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22:535–541. doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Faostat (n.d. https://www.fao.org/faostat/en/#data/FO
- 7.Gil-Pelegrín E., Peguero-Pina J.J., Sancho-Knapik D., editors. Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L. first ed. Springer; New York, NY, 2018: 2017. [Google Scholar]
- 8.Kappelle M., editor. Ecology and Conservation of Neotropical Montane Oak Forests. Springer; Berlin, Heidelberg: 2006. [DOI] [Google Scholar]
- 9.García-Villalba R., Espín J.C., Tomás-Barberán F.A., Rocha-Guzmán N.E. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food Compos. Anal. 2017;63:38–46. doi: 10.1016/j.jfca.2017.07.034. [DOI] [Google Scholar]
- 10.15.1.1.6. A faállománnyal borított erdőgazdálkodási célú erdőterület megoszlása fafajcsoportok és korosztályok szerint, december 31., (n.d.). https://www.ksh.hu/stadat_files/kor/hu/kor0004.html(accessed February 7, 2023)..
- 11.15.1.1.8. Fakitermelés az erdőgazdálkodási célú erdőterületeken fafajcsoportok szerint, (n.d.). https://www.ksh.hu/stadat_files/kor/hu/kor0006.html (accessedFebruary 7, 2023)..
- 12.Dedrie M., Jacquet N., Bombeck P.-L., Hébert J., Richel A. Oak barks as raw materials for the extraction of polyphenols for the chemical and pharmaceutical sectors: a regional case study. Ind. Crop. Prod. 2015;70:316–321. doi: 10.1016/j.indcrop.2015.03.071. [DOI] [Google Scholar]
- 13.Agarwal C., Hofmann T., Visi-Rajczi E., Pásztory Z. Low-frequency, green sonoextraction of antioxidants from tree barks of Hungarian woodlands for potential food applications. Chemical Engineering and Processing - Process Intensification. 2021;159 doi: 10.1016/j.cep.2020.108221. [DOI] [Google Scholar]
- 14.Ferreira J.P.A., Miranda I., Sousa V.B., Pereira H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa V., Ferreira J.P.A., Miranda I., Quilhó T., Pereira H. Quercus rotundifolia bark as a source of polar extracts: structural and chemical characterization. Forests. 2021;12:1160. doi: 10.3390/f12091160. [DOI] [Google Scholar]
- 16.Tian H., Zhai W., Sun K., Zhu Y., Zhou H., Wan P. Chemical composition and potential bioactivities of essential oil from Quercus mongolica bark. Arab. J. Chem. 2022;15 doi: 10.1016/j.arabjc.2022.104076. [DOI] [Google Scholar]
- 17.Nisca A., Ștefănescu R., Moldovan C., Mocan A., Mare A.D., Ciurea C.N., Man A., Muntean D.-L., Tanase C. Optimization of microwave assisted extraction conditions to improve phenolic content and in vitro antioxidant and anti-microbial activity in Quercus cerris bark extracts. Plants. 2022;11:240. doi: 10.3390/plants11030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanase C., Nicolescu A., Nisca A., Ștefănescu R., Babotă M., Mare A.D., Ciurea C.N., Man A. Biological activity of bark extracts from northern red oak (Quercus rubra L.): an antioxidant, antimicrobial and enzymatic inhibitory evaluation. Plants. 2022;11:2357. doi: 10.3390/plants11182357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz P., Heinrich M., Garcia-Käufer M., Grunewald F., Messerschmidt S., Herrick A., Gruber K., Beckmann C., Knoedler M., Huber R., Steinborn C., Stintzing F.C., Gründemann C. Constituents from oak bark (Quercus robur L.) inhibit degranulation and allergic mediator release from basophils and mast cells in vitro. J. Ethnopharmacol. 2016;194:642–650. doi: 10.1016/j.jep.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Vovk I., Simonovska B., Andrensek S., Vuorela H., Vuorela P. Rotation planar extraction and rotation planar chromatography of oak (Quercus robur L.) bark. J. Chromatogr. A. 2003;991:267–274. doi: 10.1016/s0021-9673(03)00271-1. [DOI] [PubMed] [Google Scholar]
- 21.Morel I., Lescoat G., Cogrel P., Sergent O., Pasdeloup N., Brissot P., Cillard P., Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 22.Nakao M., Takio S., Ono K. Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry. 1998;49:2379–2382. doi: 10.1016/s0031-9422(98)00333-1. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz Y. Novel uses of catechins in foods. Trends Food Sci. Technol. 2006;17:64–71. doi: 10.1016/j.tifs.2005.10.005. [DOI] [Google Scholar]
- 24.Bae J., Kim N., Shin Y., Kim S.-Y., Kim Y.-J. Activity of catechins and their applications. Biomedical Dermatology. 2020;4:8. doi: 10.1186/s41702-020-0057-8. [DOI] [Google Scholar]
- 25.Reimann H.J., Lorenz W., Fischer M., Frölich R., Meyer H.J., Schmal A. Histamine and acute haemorrhagic lesions in rat gastric mucosa: prevention of stress ulcer formation by (+)-catechin, an inhibitor of specific histidine decarboxylase in vitro. Agents Actions. 1977;7:69–73. doi: 10.1007/BF01964883. [DOI] [PubMed] [Google Scholar]
- 26.Nath S., Bachani M., Harshavardhana D., Steiner J.P. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J. Neurovirol. 2012;18:445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Stautemas J., Omondi Onyango S., De Mey M., Duchi D., Tuenter E., Hermans N., Calders P., Van de Wiele T. Human gut microbiota stratified by (+)-catechin metabolism dynamics reveals colon region-dependent metabolic profile. Food Chem. 2023;408 doi: 10.1016/j.foodchem.2022.135203. [DOI] [PubMed] [Google Scholar]
- 28.Takano T., Murakami T., Kamitakahara H., Nakatsubo F. Mechanism of formaldehyde adsorption of (+)-catechin. J. Wood Sci. 2008;54:329–331. doi: 10.1007/s10086-008-0946-8. [DOI] [Google Scholar]
- 29.Ono K., Nakao M., Toyota M., Terashi Y., Yamada M., Kohno T., Asakawa Y. Catechin production in cultured Polygonum hydropiper cells. Phytochemistry. 1998;49:1935–1939. doi: 10.1016/S0031-9422(98)00426-9. [DOI] [Google Scholar]
- 30.Bhadange Y.A., Saharan V.K., Sonawane S.H., Boczkaj G. Intensification of catechin extraction from the bark of Syzygium cumini using ultrasonication: optimization, characterization, degradation analysis and kinetic studies. Chemical Engineering and Processing - Process Intensification. 2022;181 doi: 10.1016/j.cep.2022.109147. [DOI] [Google Scholar]
- 31.Ghasemzadeh-mohammadi V., Zamani B., Afsharpour M., Mohammadi A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: an optimization study by the IV-optimal design. Food Sci. Biotechnol. 2017;26:1281–1290. doi: 10.1007/s10068-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z., Huang D., Tang Z., Deng C. Microwave-assisted extraction followed by CE for determination of catechin and epicatechin in green tea: electrodriven Separations. J. Sep. Science. 2010;33:1079–1084. doi: 10.1002/jssc.200900647. [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Thilakarathna W.P.D.W., Astatkie T., Rupasinghe H.P.V. Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules. 2020;10:243. doi: 10.3390/biom10020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananingsih V.K., Sharma A., Zhou W. Green tea catechins during food processing and storage: a review on stability and detection. Food Res. Int. 2013;50:469–479. doi: 10.1016/j.foodres.2011.03.004. [DOI] [Google Scholar]
- 35.Hofmann T., Nebehaj E., Albert L. The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J. Chromatogr. A. 2015;1393:96–105. doi: 10.1016/j.chroma.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann T., Nebehaj E., Stefanovits-Bányai É., Albert L. Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Ind. Crop. Prod. 2015;77:375–381. doi: 10.1016/j.indcrop.2015.09.008. [DOI] [Google Scholar]
- 37.Makk Á.N., Rétfalvi T., Hofmann T. Utilization of oak (Quercus petreae (matt.) Liebl.) bark for anaerobic digested biogas production. Acta Silvatica Lignaria Hung.: An International Journal In Forest, Wood And Environmental Sciences. 2017;13:125–134. [Google Scholar]
- 38.Shannon L.M., Kay E., Lew J.Y. Peroxidase isozymes from horseradish roots: I. Isolation and physical properties. J. Biol. Chem. 1966;241:2166–2172. doi: 10.1016/S0021-9258(18)96680-9. [DOI] [PubMed] [Google Scholar]
- 39.Flurkey W.H., Jen J.J. Peroxidase and polyphenol oxidase activities in developing peaches. J. Food Sci. 1978;43:1826–1828. doi: 10.1111/j.1365-2621.1978.tb07424.x. [DOI] [Google Scholar]
- 40.Jerez M., Touriño S., Sineiro J., Torres J.L., Núñez M.J. Procyanidins from pine bark: relationships between structure, composition and antiradical activity. Food Chem. 2007;104:518–527. doi: 10.1016/j.foodchem.2006.11.071. [DOI] [Google Scholar]
- 41.Hofmann T., Albert L., Rétfalvi T., Visi-Rajczi E., Brolly G. TLC analysis of the in-vitro reaction of beech (Fagus sylvatica L.) wood enzyme extract with catechins. JPC - Journal of Planar Chromatography - Modern TLC. 2008;21:83–88. doi: 10.1556/jpc.21.2008.2.2. [DOI] [Google Scholar]
- 42.Martinez M.V., Whitaker J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995;6:195–200. doi: 10.1016/S0924-2244(00)89054-8. [DOI] [Google Scholar]
- 43.Jiménez-Atiénzar M., Cabanes J., Gandía-Herrero F., García-Carmona F. Kinetic analysis of catechin oxidation by polyphenol oxidase at neutral pH. Biochem. Biophys. Res. Commun. 2004;319:902–910. doi: 10.1016/j.bbrc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Sheng J., Yang F., Hu Q. Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea. J. Food Eng. 2007;78:687–692. [Google Scholar]
- 45.Juarez-Enriquez E., Salmerón I., Gutierrez-Mendez N., Ortega-Rivas E. Ultraviolet irradiation effect on apple juice bioactive compounds during shelf storage. Foods. 2016;5:10. doi: 10.3390/foods5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chutintrasri B., Noomhorm A. Thermal inactivation of polyphenoloxidase in pineapple puree. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2006;39:492–495. doi: 10.1016/j.lwt.2005.04.006. [DOI] [Google Scholar]
- 47.Noci F., Riener J., Walkling-Ribeiro M., Cronin D.A., Morgan D.J., Lyng J.G. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple Juice. J. Food Eng. 2008;85:141–146. doi: 10.1016/j.jfoodeng.2007.07.011. [DOI] [Google Scholar]
- 48.Vámos‐Vigyázó L., Haard N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. CRC Crit. Rev. Food Sci. Nutr. 1981;15:49–127. doi: 10.1080/10408398109527312. [DOI] [PubMed] [Google Scholar]
- 49.Visi-Rajczi E., Hofmann T., Albert L., Mátyás C. Tracing the acclimation of European beech (Fagus sylvatica L.) populations to climatic stress by analyzing the antioxidant system. IForest. 2021;14:95–103. doi: 10.3832/ifor3542-013. [DOI] [Google Scholar]
- 50.III J.A.Z. US6344226B1; 2002. Process for Producing an Extract of an Accelerated Oak Aged Alcoholic Concentrate.https://patents.google.com/patent/US6344226B1/en accessed June 5, 2023. [Google Scholar]
- 51.Gao M., Liu C.-Z. Comparison of techniques for the extraction of flavonoids from cultured cells of saussurea medusa maxim. World J. Microbiol. Biotechnol. 2005;21:1461–1463. doi: 10.1007/s11274-005-6809-1. [DOI] [Google Scholar]
- 52.Bouras M., Chadni M., Barba F.J., Grimi N., Bals O., Vorobiev E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crop. Prod. 2015;77:590–601. doi: 10.1016/j.indcrop.2015.09.018. [DOI] [Google Scholar]
- 53.Albuquerque B.R., Prieto M.A., Barreiro M.F., Rodrigues A., Curran T.P., Barros L., Ferreira I.C.F.R. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crop. Prod. 2017;95:404–415. doi: 10.1016/j.indcrop.2016.10.050. [DOI] [Google Scholar]
- 54.Rahim A.A., Nofrizal S., Saad B. Rapid tea catechins and caffeine determination by HPLC using microwave-assisted extraction and silica monolithic column. Food Chem. 2014;147:262–268. doi: 10.1016/j.foodchem.2013.09.131. [DOI] [PubMed] [Google Scholar]
- 55.Gong Z., Chen S., Gao J., Li M., Wang X., Lin J., Yu X. [Isolation and purification of seven catechin compounds from fresh tea leaves by semi-preparative liquid chromatography] Se Pu. 2017;35:1192–1197. doi: 10.3724/SP.J.1123.2017.08002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.