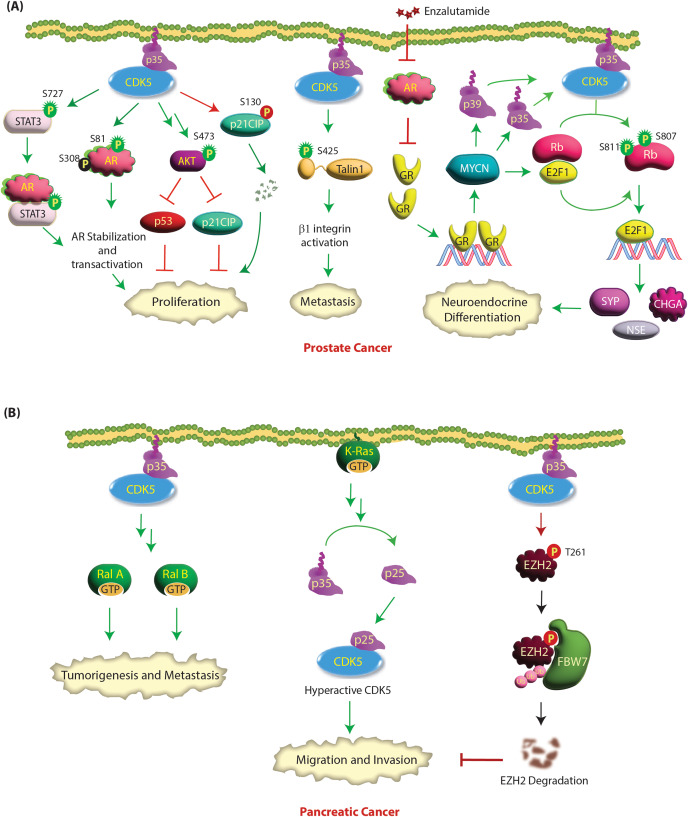
Fig. 2.
(A) CDK5 facilitates highly oncogenic phenotypes in prostate cancer. CDK5 can simultaneously affect numerous targets and promote PCa progression. CDK5/p35 overexpression in prostate tumor cells increases the phosphorylation of talin1 at S425, which induces a conformational change resulting in talin1 activation. Phosphorylated talin1 then binds to the cytoplasmic tail of β1 integrin, leading to β1 integrin activation and downstream integrin signaling. This leads to increased cell survival, adhesion, motility and metastatic potential of PCa cells. CDK5 also activates STAT3 and AR proteins by phosphorylation at specific sites. Activation of STAT3 or AR causes prostate cancer proliferation. CDK5 indirectly activates AKT resulting in p53 and p21CIP downregulation and cell cycle progression. CDK5 directly phosphorylates p21CIP at S130, which degrades it, promoting oncogenesis. Enzalutamide treatment upregulates GR signaling, which transcriptionally increases MYCN levels. MYCN increases p35, p39 and E2F1 transcription. p35 and p39 binding activates CDK5, which phosphorylates Rb at S807 and S811, releasing E2F1, which in turn transcriptionally upregulates the NEPC genes, leading to neuroendocrine differentiation. Green and red circles show activating and inactivating phosphorylation events, respectively. Green and red arrows represent activating and inactivating pathways, respectively. (B) CDK5 signaling in pancreatic cancer. K-Ras activates CDK5 by promoting the cleavage of p35 to p25, although the exact mechanism is not known. CDK5 induces cell migration and invasion through activation of RalA and RalB in pancreatic cancer. CDK5 directly phosphorylates EZH2 at T261, causing its ubiquitylation by FBW7, which in turn inhibits pancreatic cancer cell migration and invasion