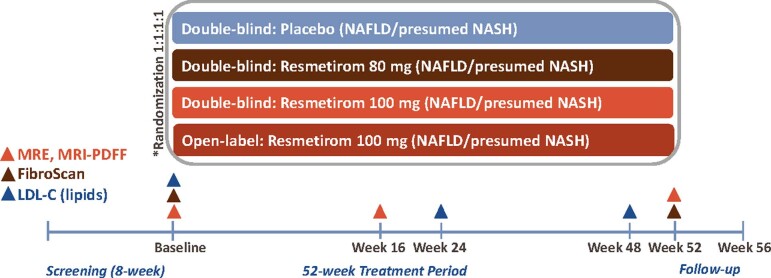
Extended Data Fig. 3. Study Design.
*Randomization was stratified by type 2 diabetes status and by history of documented atherosclerotic cardiovascular disease. The first 30 patients enrolled in the MAESTRO-NAFLD-1 trial were enrolled in the open-label 100 mg resmetirom (including any patients taking thyroxine >75 mcg). After the first 30 patients were enrolled in the open-label 100 mg resmetirom arm, subsequent patients were randomized 1:1:1:1 to 3 double-blind arms (100 mg resmetirom, 80 mg resmetirom, or placebo) or the open-label 100 mg resmetirom arm. Randomization to the open-label arm was discontinued when the target number of patients was achieved (July 1, 2020). Thereafter, eligible patients were randomized 1:1:1 to the 3 double-blind arms (100 mg resmetirom, 80 mg resmetirom, or placebo).