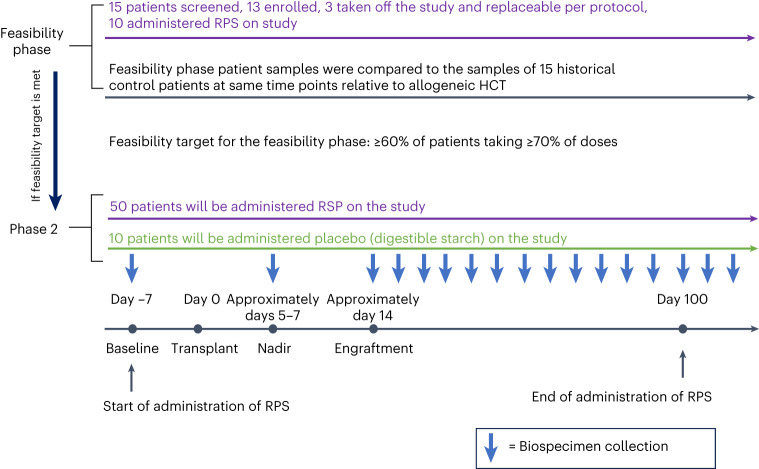
Fig. 1. Schema for the clinical trial and longitudinal biospecimen collection.
Study participants received 20 g RPS orally daily for the first 3 days followed by twice daily, from day −7 through to day 100 after allogeneic HCT. Stool and blood specimens were collected from the study participants at baseline before conditioning (day −7), nadir (approximately days 5–7), engraftment (approximately day 14) and day 100. Stool samples were collected weekly after allogeneic HCT when possible. Samples were compared to samples obtained from past historical controls not receiving the dietary intervention at the same time points relative to allogeneic HCT. After the feasibility phase, an additional 50 individuals will be enrolled on phase 2 of the trial to receive RPS on the same schedule noted above and ten individuals will be enrolled to receive isocaloric, nonresistant starch placebo, who will serve as contemporaneous controls (5:1 randomization). Stool and blood specimens will be collected on the same schedule as the feasibility phase.