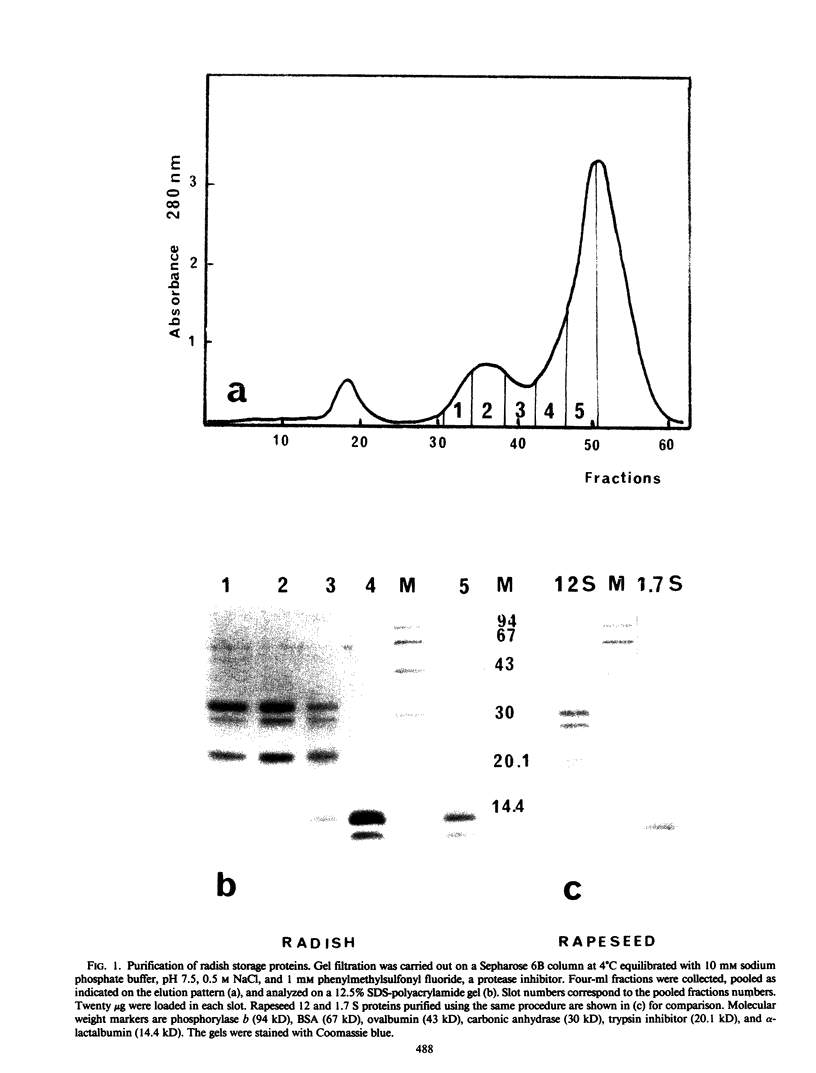
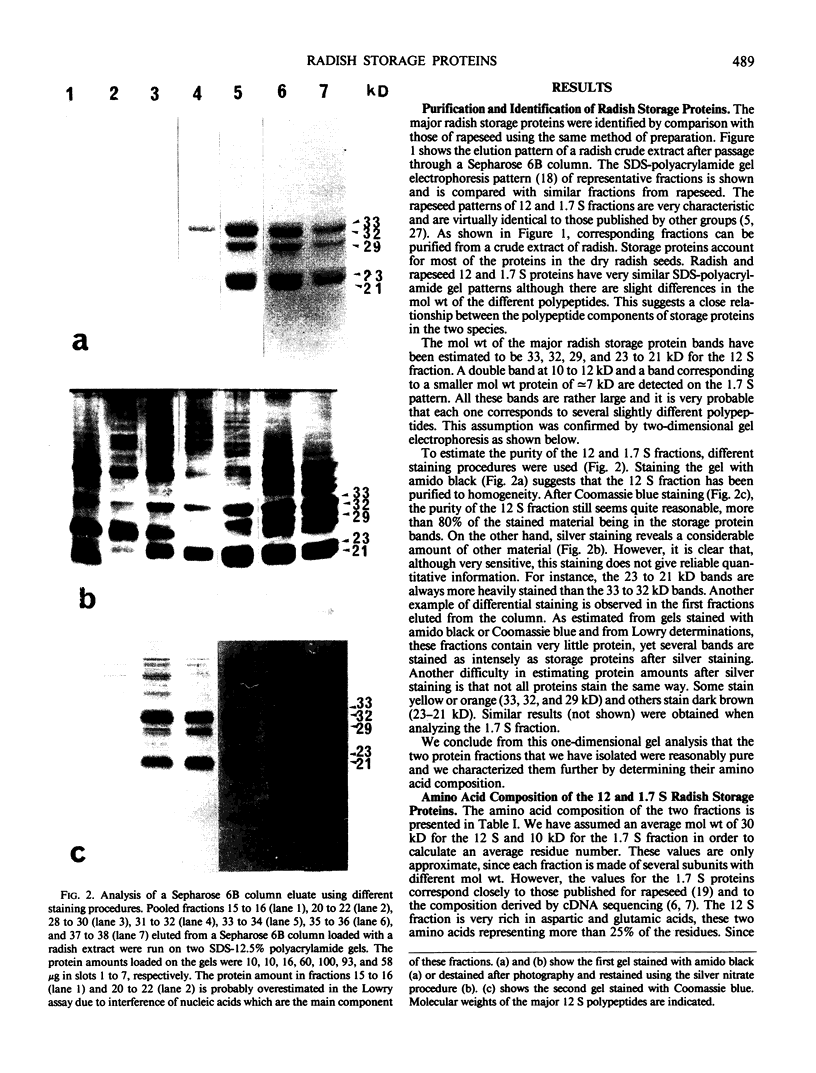
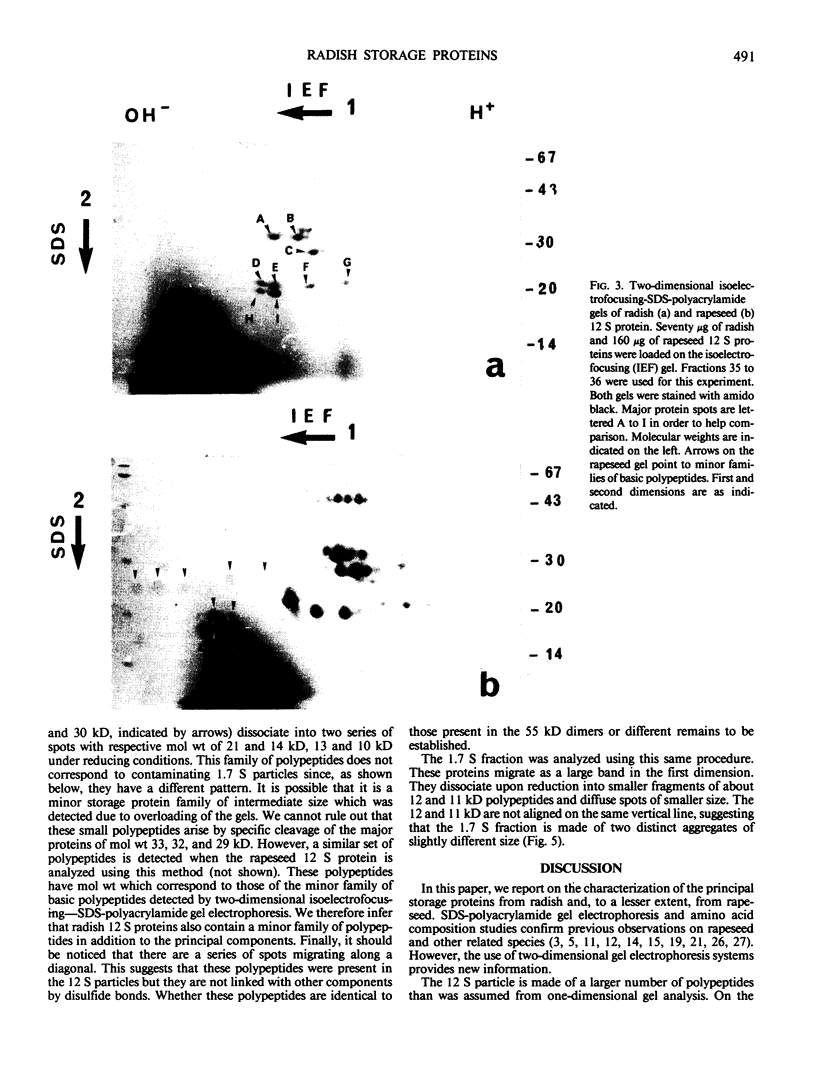
Abstract
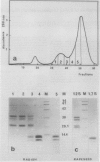
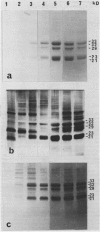
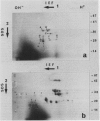
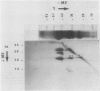
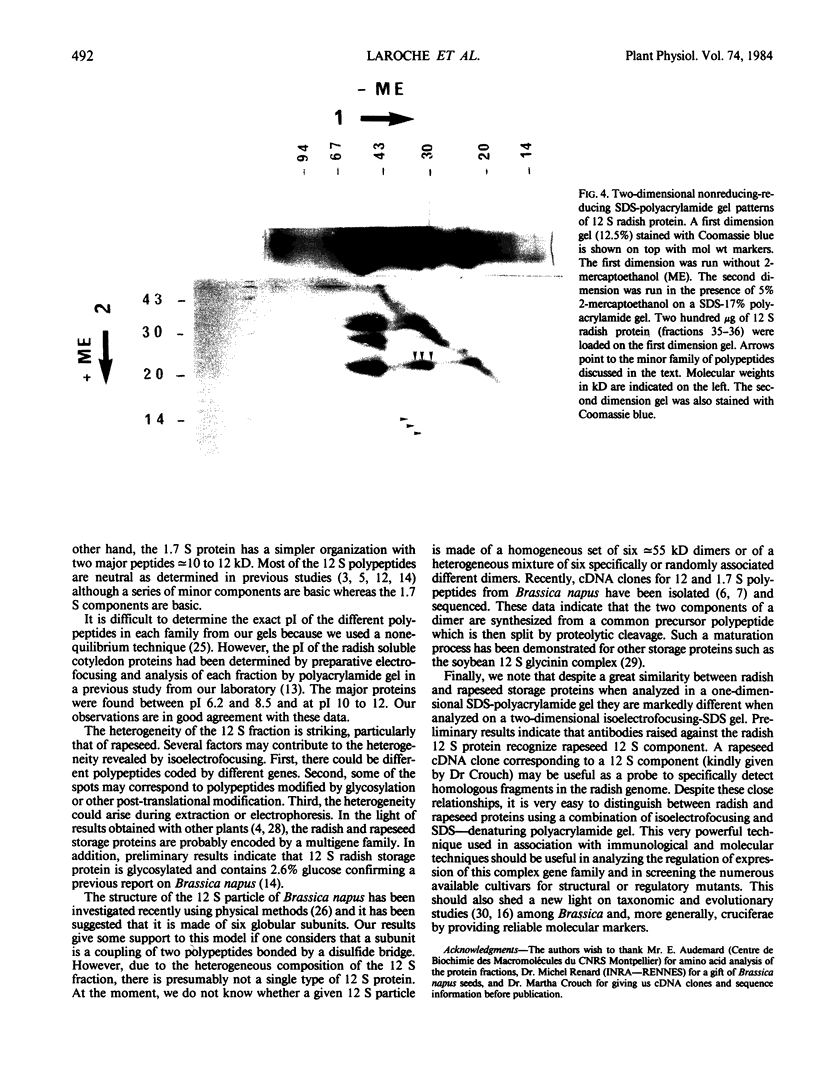
Radish (Raphanus sativus cv Rond rose à bout blanc Vilmorin) seeds, as other cruciferae oil seeds, contain two major types of storage protein aggregates which can be separated by gel filtration into 12 and 1.7 Svedberg fractions. These two fractions have been characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, amino acid composition, and two bidimensional gel electrophoresis systems. These results were compared with those obtained with rapeseed storage proteins. Radish 12 Svedberg particles are made of a series of nine major polypeptides ranging from 33 to 30 kilodaltons. These polypeptides present charge heterogeneity. The 12 Svedberg particle is made of six subunits ≃ 55 kilodaltons. Each subunit is a couple of two polypeptides linked by a disulfide bridge. The 1.7 Svedberg particle has a simpler composition. It is made of two polypeptides of 10 and 12 kilodaltons and smaller peptides of ≃ 7 kilodaltons. Twelve and 1.7 Svedberg particles also differ in their amino acid composition, the 1.7 Svedberg being particularly rich in glutamic acid and proline. Its components are basic. The organization of the rapeseed storage protein is similar but more complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspart L., Cooke R., Delseny M. Stability of polyadenylic and polyadenylated ribonucleic acids in radish (Raphanus sativus) seedlings. Biochim Biophys Acta. 1979 Aug 29;564(1):43–54. doi: 10.1016/0005-2787(79)90187-4. [DOI] [PubMed] [Google Scholar]
- Bhatty R. S., McKenzie S. L., Finlayson A. J. The proteins of rapeseed (Brassica napus L.) soluble in salt solutions. Can J Biochem. 1968 Oct;46(10):1191–1197. doi: 10.1139/o68-178. [DOI] [PubMed] [Google Scholar]
- Goding L. A., Bhatty R. S., Finlayson A. J. The characterization of the 12 S "globulin" from rapeseed and its glycoprotein component. Can J Biochem. 1970 Oct;48(10):1096–1103. doi: 10.1139/o70-173. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Janson J. C. Studies on Brassica seed proteins. I. The low molecular weight proteins in rapeseed. Isolation and characterization. Biochim Biophys Acta. 1972 Aug 31;278(1):175–183. [PubMed] [Google Scholar]
- Meyer Y., Chartier Y. Hormonal Control of Mitotic Development in Tobacco Protoplasts: TWO-DIMENSIONAL DISTRIBUTION OF NEWLY-SYNTHESIZED PROTEINS. Plant Physiol. 1981 Dec;68(6):1273–1278. doi: 10.1104/pp.68.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Plietz P., Damaschun G., Müller J. J., Schwenke K. D. The structure of 11-S globulins from sunflower and rape seed. A small-angle X-ray scattering study. Eur J Biochem. 1983 Feb 1;130(2):315–320. doi: 10.1111/j.1432-1033.1983.tb07154.x. [DOI] [PubMed] [Google Scholar]
- Tumer N. E., Thanh V. H., Nielsen N. C. Purification and characterization of mRNA from soybean seeds. Identification of glycinin and beta-conglycinin precursors. J Biol Chem. 1981 Aug 25;256(16):8756–8760. [PubMed] [Google Scholar]