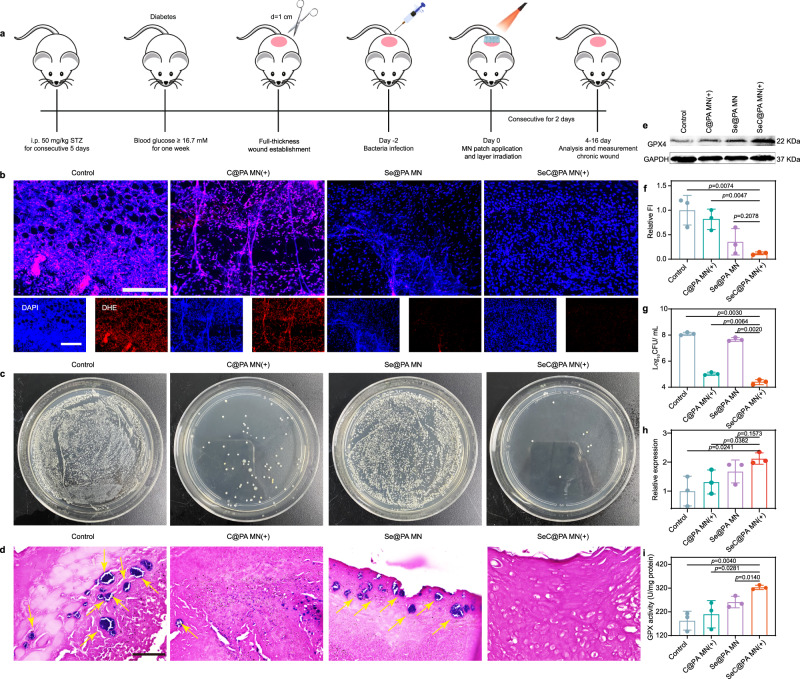
Fig. 9. SeC@PA MN for reducing wound inflammation and bacteria to promote angiogenesis.
a Schematic illustration of the experimental procedure for treating biofilms-infected mice with diabetic. b, f Representative immunofluorescence images of RS staining and relative fluorescence intensity of RS at day 4 (n = 3 biologically independent samples; mean ± SD). Scale bar is 100 μm. c, g Representative photographs of bacterial culture from the skin tissue of diabetic mouse wounds infected with biofilms and bactericidal results at day 4 characterized by the standard plate counting assay (n = 3 biologically independent samples; mean ± SD). d Gram-staining images of the skin tissue of diabetic mice wounds infected with biofilms at day 4. The yellow arrows represent bacteria. Scale bar is 50 μm. Three independent experiments were performed and representative results are shown. e, h Expression levels of GPX4 from the wound tissues of diabetic mice infected with biofilms at day 8 determined by western blotting (n = 3 biologically independent samples; mean ± SD). The experiment in e was repeated three times with similar results. i Activity of GPX4 from the wound tissue of diabetic mice infected with biofilms at day 8 (n = 3 biologically independent samples; mean ± SD). Statistical significance was analyzed via one-way ANOVA with a Tukey post-hoc test. Source data are provided as a Source Data file. Control: blank microneedle; C@PA MN(+): microneedle containing Ce6-PDA-LA nanoparticles under 660 nm irradiation (0.2 W/cm2) for 3 min; Se@PA MN: microneedle containing Se-PDA-LA nanoparticles; SeC@PA MN(+): microneedle containing Se-Ce6-PDA-LA nanoparticles under 660 nm irradiation (0.2 W/cm2) for 3 min.