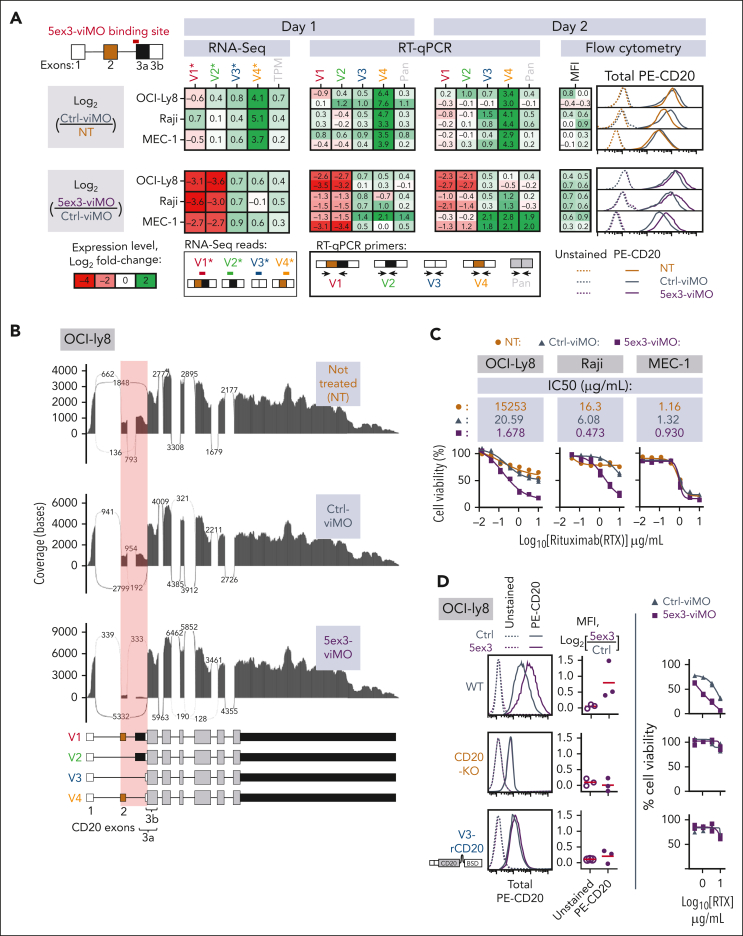
Figure 5.
Shifting CD20 splicing toward V3 and V4 increases CD20 protein expression and rituximab-mediated cytotoxicity. (A-C) CD20 mRNA and protein measurements in OCI-Ly8, Raji, and MEC-1 cells treated with either the solvent control (no-Morpholino treatment [NT]), Ctrl-viMO, or 5ex3-viMO. (D) The same experiment performed on OCI-Ly8 variants with the intact MS4A1 gene (WT, top), knocked out MS4A1 gene (CD20-KO, middle), or knocked out MS4A1 gene replaced with the V3 expression cassette (V3-rCD20, bottom). CD20 RNA levels were quantified using RNA-seq (leftmost heat map in panel A and sashimi plot in panel B) and RT-qPCR (middle heatmaps in panel A). For the RNA-seq data in panel A, V1∗-4∗ are reads that mapped to the unique exon junctions found in each 5′-UTR variant of CD20, color-coded as shown in the RNA-seq reads panel. The MFI of total CD20 protein was determined using flow cytometry (rightmost heat map in panel A, top panel in panel C, and left and middle panels in panel D). Representative histograms are shown on the far right of panel A and far left of panel D. At the bottom panel of panel C and the right panel of panel D are shown cells that were additionally treated with increasing concentrations of rituximab (RTX), and cell viability (plotted) was measured using the WST-1 assay. All values are normalized to the “no rituximab Ctrl.” In panels A,C-D, each dot in a graph or value in a heat map represents independent experiments (N ≥ 2), with mean values indicated by red lines in the graphs.