Abstract
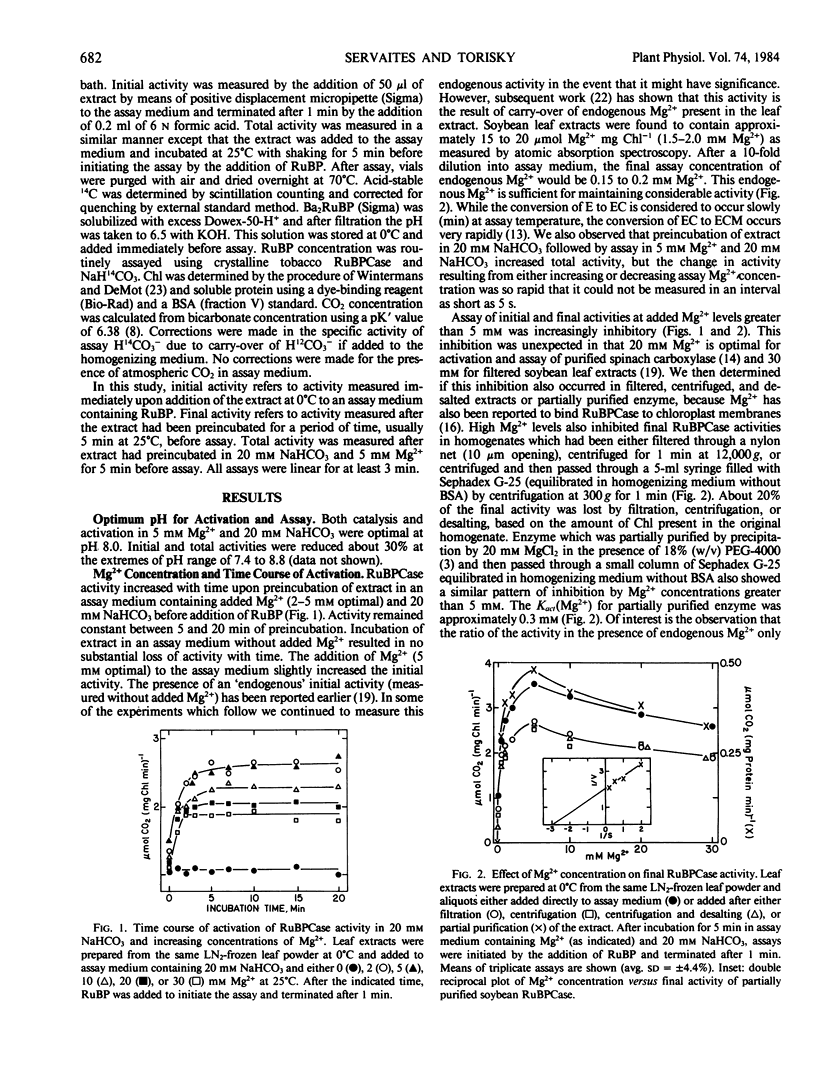
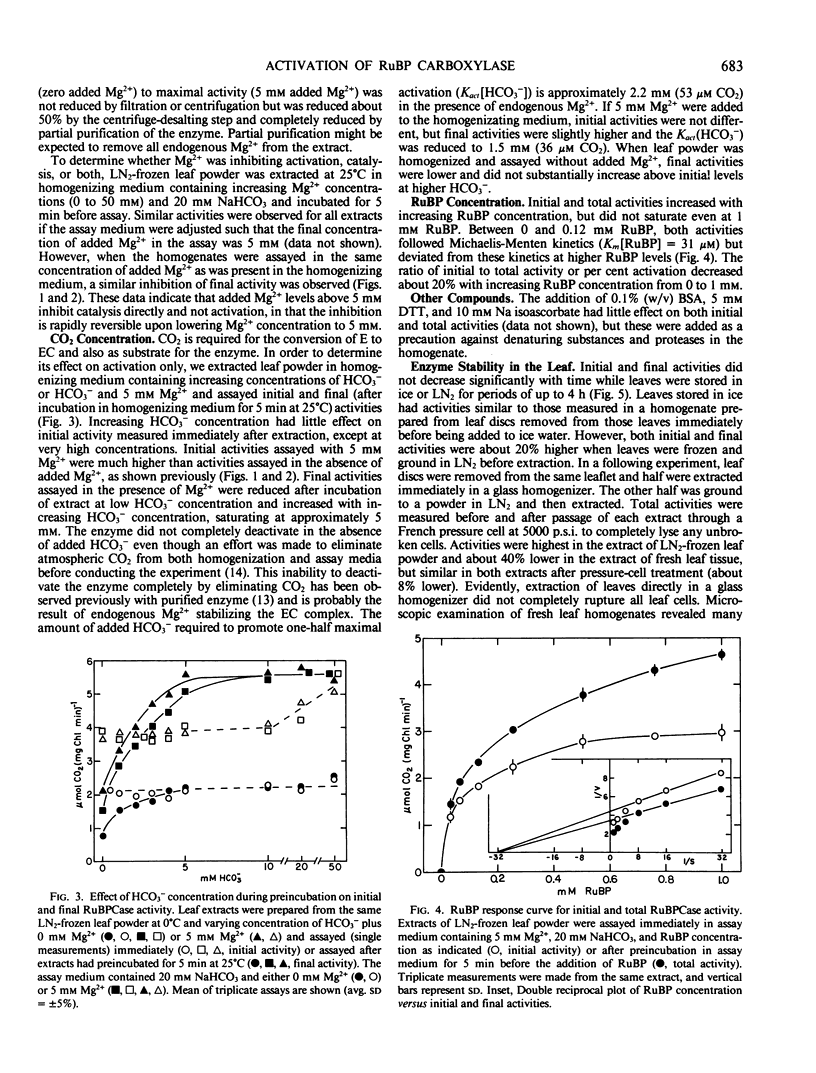
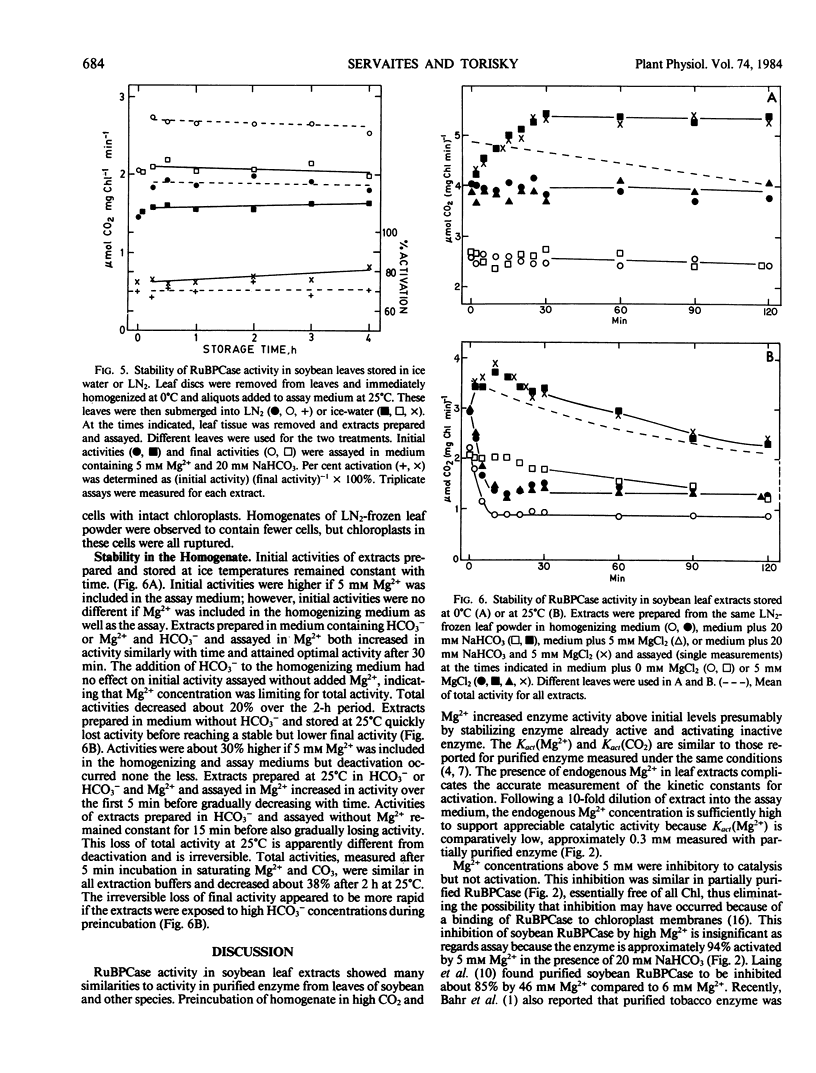
Conditions for extraction and assay of ribulose-1,5-bisphophate carboxylase present in an in vivo active form (initial activity) and an inactive form able to be activated by Mg2+ and CO2 (total activity) were examined in leaves of soybean, Glycine max (L.) Merr. cv Will. Total activity was highest after extracts had preincubated in NaHCO3 (5 millimolar saturating) and Mg2+ (5 millimolar optimal) for 5 minutes at 25°C or 30 minutes at 0°C before assay. Initial activity was about 70% of total activity. Kact (Mg2+) and Kact (CO2) were approximately 0.3 millimolar and 36 micromolar, respectively. The carry-over of endogenous Mg2+ in the leaf extract was sufficient to support considerable catalytic activity. While Mg2+ was essential for both activation and catalysis, Mg2+ levels greater than 5 millimolar were increasingly inhibitory of catalysis. Similar inhibition by high Mg2+ was also observed in filtered, centrifuged, or desalted extracts and partially purified enzyme. Activities did not change upon storage of leaves for up to 4 hours in ice water or liquid nitrogen before homogenization, but were about 20% higher in the latter. Activities were also stable for up to 2 hours in leaf extracts stored at 0°C. Initial activity quickly deactivated at 25°C in the absence of high CO2. Total activity slowly declined irreversibly upon storage of leaf homogenate at 25°C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hatch A. L., Jensen R. G. Regulation of ribulose-1,5-bisphosphate carboxylase from tobacco: changes in pH response and affinity for CO2 and Mg2+ induced by chloroplast intermediates. Arch Biochem Biophys. 1980 Dec;205(2):587–594. doi: 10.1016/0003-9861(80)90142-3. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A steady-state kinetic study on the catalytic mechanism of ribulose bisphosphate carboxylase from soybean. Arch Biochem Biophys. 1980 Jul;202(2):592–600. doi: 10.1016/0003-9861(80)90466-x. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Ogren W. L., Hageman R. H. Bicarbonate stabilization of ribulose 1,5-diphosphate carboxylase. Biochemistry. 1975 May 20;14(10):2269–2275. doi: 10.1021/bi00681a035. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H. Evidence for the existence of discrete activator and substrate sites for CO2 on ribulose-1,5-bisphosphate carboxylase. J Biol Chem. 1979 Jul 10;254(13):5599–5601. [PubMed] [Google Scholar]
- McNeil P. H., Walker D. A. The effect of magnesium and other ions on the distribution of ribulose 1,5-bisphosphate carboxylase in chloroplast extracts. Arch Biochem Biophys. 1981 Apr 15;208(1):184–188. doi: 10.1016/0003-9861(81)90138-7. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Measurement and preservation of the in vivo activation of ribulose 1,5-bisphosphate carboxylase in leaf extracts. Plant Physiol. 1982 May;69(5):1165–1168. doi: 10.1104/pp.69.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


