Figure 4.
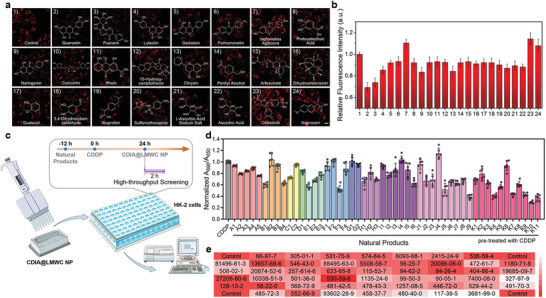
HTS of natural products with CDIA@LMWC NP in living HK‐2 cells for AKI. a) HK‐2 cells were pretreated with 50 µm various natural antioxidants for 12 h before 75 µm CDDP for 24 h, and then images were obtained by high‐content analysis after incubation with 20 µg mL−1 CDIA@LMWC NP at 37 °C for 2 h. Scale bar: 20 µm. b) Quantification performed on the relative ratio of fluorescence intensity shown in (a). Data represent mean ± SD (n = 3). c) Diagram of the strategy for HTS of antioxidant natural products using microplate reader. d) Antioxidant effects of natural products on •OH scavenging by utilizing CDIA@LMWC NP. A: coumarins, A1: psoralen, A2: 6,7‐dihydroxycoumarin, A3: esculin hydrate, A4: fraxetin, A5: 6‐hydroxycoumarin; B: sesquiterpenes, B1: dihydroartemisinin, B2: curdione, B3: alantolactone, B4: artesunate; C: monoterpenes, C1: catalpol, C2: perillyl alcohol; D: diterpenes, D1: andrographolide; E: triterpenes, E1: oleanolic acid, E2: saikosaponin D, E3: ginsenoside Re; F: others, F1: cantharidin, F2: diosbulbin B, F3: astaxanthin, F4: limonin; G: steroids, G1: ursodeoxycholic acid, G2: testosterone; H: quinones, H1: tanshinone IIA, H2: 5‐hydroxy‐2‐methyl‐1,4‐naphthoquinon, H3: rhein; I: alkaloids, I1: berberine, I2: sinomenine, I3: piperine, I4: rutecarpine, I5: capsaicin, I6: 10‐hydroxy camptothecin; J: phenolics, J1: polydatin, J2: salidroside, J3: resveratrol, J4: sinapic acid, J5: ferulic acid, J6: protocatechuic acid, J7: guaiacol, J8: p‐hydroxy‐cinnamic acid, J9: chlorogenic acid; K: flavones, K1: (‐)‐epicatechin gallate, K2: genistein, K3: myricetin, K4: luteolin, K5: formononetin, K6: isoflavone aglycone, K7: naringenin, K8: curcumin, K9: chrysin, K10: Que, K11: Pue. Data represent mean ± SD (n = 6). e) The CAS numbers of the natural compounds corresponding to each microplate for antioxidant screening.
