Figure 7.
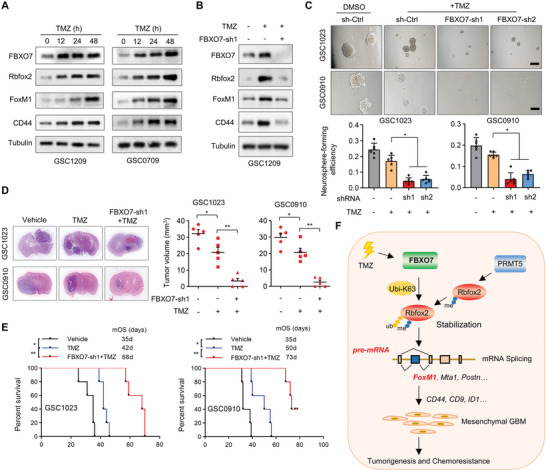
FBXO7 mediates TMZ‐induced mesenchymal properties, and depleting FBXO7 sensitizes GBM to chemotherapy. A) GSC1209 cells were treated with TMZ (100 µm) for the indicated time intervals, and the levels of FBXO7, Rbfox2, FoxM1, and CD44 were detected by immunoblotting. B) GSC1209 cells expressing FBXO7 shRNA were treated with TMZ for 48 h, and the expression of FBXO7, Rbfox2, FoxM1, and CD44 were detected by immunoblotting. C) GSC1023 and GSC0910 cells expressing FBXO7 shRNAs or control shRNA were treated with TMZ, and neurosphere formation was assessed. Scale bar, 500 µm. The neurosphere formation efficiency (spheres/cells plated) was quantified (mean ± S.E.M., n = 6 independent experiments, two‐tailed Student's t‐test). *P<0.05. D) GSC1023 and GSC0910 cells expressing FBXO7 shRNAs were intracranially injected into nude mice (5 × 105 cells per mouse). One day after cell injection, mice were intraperitoneally injected with TMZ (20 mg kg per d) every other day for 4 weeks. Thirty days after injection, the mice were humanely killed and tumor growth was assessed. The H&E‐stained sections show representative tumor xenografts. Tumor volumes were calculated (mean ± S.D., n = 5 mice for each group, One‐way ANOVA test). *P<0.05, **P<0.01. E) GSC1023 and GSC0910 cells were intracranially injected nude mice, and mice were treated as in (D). The survival of mice was evaluated (n = 5 mice for each group, Kaplan–Meier model with two‐sided log‐rank test). *P<0.05, **P<0.01. F) Illustration of the FBXO7‐Rbfox2 axis in the regulation of GBM MES transformation, tumorigenesis, and chemoresistance. FBXO7 interacts with and ubiquitinates Rbfox2 through K63‐linked ubiquitin chains after its arginine dimethylation by PRMT5. FBXO7 stabilizes Rbfox2 and controls Rbfox2‐mediated splicing of MES‐related genes, including FoxM1, Mta1, and Postn. FBXO7‐induced exon Va inclusion of FoxM1 induces its nuclear translocation and stabilization, leading to upregulation of CD44, CD9, and ID1, and thus promotes GBM MES transformation and tumorigenesis.
