Abstract
Introduction and importance
May-Thurner Syndrome (MTS) is an uncommon etiology of left common iliac vein thrombosis due to arterial compression. In this report, we describe a case of MTS with severe occlusion of the left common iliac vein in the context of a previously undiagnosed pancreatic cancer. We detail the endovascular resolution of the iliac vein compression and show long-term patency.
Case presentation
A 33-year-old woman on oral contraceptive pills presented with extensive thrombosis of the left common iliac vein extending cephalad into the lower IVC and inferiorly to the femoral vein. The thrombus was refractory to therapeutic heparin. Mechanical thrombectomy removed the occluding thrombus. Intravenous ultrasound identified severe compression of the left common iliac vein by the right common iliac artery. Angioplasty and stenting provided complete resolution of the lesion. Imaging and hematologic workup revealed a pancreatic malignancy and concomitant hypercoagulable state that likely precipitated the patient's presentation.
Clinical discussion
Endovascular intervention provided complete resolution of severe iliac vein compression. Patency was maintained at 6-month follow-up. Research suggests that the anatomical lesion predisposing individuals to MTS is relatively common despite infrequent occurrence of the syndrome. This case highlights the importance of a high clinical suspicion for associated hypercoagulable states when MTS is discovered.
Conclusion
There is limited research exploring the relationship between severity of iliac vein compression and endovascular treatment outcome. This case documents endovascular resolution of a severe lesion with maintained patency.
Keywords: May-Thurner syndrome, Iliac vein, Vascular interventional radiology, Case report
Highlights
-
•
Extensive thrombosis and severe iliac vein stenosis can be resolved endovascularly.
-
•
Endovascular resolution of iliac vein stenosis can achieve long-term patency.
-
•
Patients with May-Thurner syndrome (MTS) should be approached with a high clinical suspicion for hypercoagulable states.
-
•
Predisposing factors for MTS include pregnancy, oral contraceptive use, immobility, the postpartum period, and malignancy.
1. Introduction
May-Thurner Syndrome (MTS) is a unique anatomic variant in which the left common iliac vein is compressed between the lower lumbar spine and an overlying right common iliac artery [1,2]. It is most commonly observed in young-to-middle-aged women and carries a predilection for the development of deep vein thrombosis (DVT). Indeed, MTS is thought to affect approximately 2–5 % of patients with DVT [2].
Endovascular intervention is the first-line treatment for MTS, and current management recommendations include a combination of anticoagulation, thrombectomy, and venous angioplasty and/or stenting [3]. However, there remain open questions about the optimal management of MTS. MTS exhibits heterogeneity in clinical presentation and severity of venous stenosis [[3], [4], [5]]. It also includes multiple anatomical variants [4,6,7]. Thus, despite increasing interest [5], the relationships between anatomy, degree of stenosis, presentation and procedural outcomes remains unclear.
In addition, the prevalence of the anatomical May-Thurner lesion is high relative to the incidence of symptomatic MTS [8]. Underlying pro-thrombotic conditions are thought to play an important role in precipitating MTS [2,8]. Prolonged immobility, pregnancy, and oral contraceptive use are known risk factors but do not capture all patient presentations, suggesting that other predisposing conditions may play a role.
Here we present a case that begins to address these two points. We describe a woman with an extensive DVT found to have a complete stenosis of the common iliac vein secondary to right common iliac artery compression. Endovascular intervention fully resolved both the thrombus and stenosis, highlighting the utility of endovascular management in severe disease. Subsequent work-up suggested that this patient was predisposed to developing MTS due to underlying pancreatic cancer and oral contraceptive use.
2. Case presentation
A 33-year-old woman on estrogen-containing oral contraceptive pills presented to an outside emergency department with 1-week history of left leg swelling, redness, and pain. Left lower extremity venous duplex ultrasound revealed deep vein thrombosis extending from left common iliac vein into the inferior vena cava. She was started on therapeutic heparin and transferred to our tertiary care center for further management.
The patient's symptoms did not improve on conservative therapy with systemic anticoagulation. She subsequently underwent CT Venogram that demonstrated thrombus extending into the lower IVC from the left common iliac vein and inferiorly involving the left external iliac vein, left internal iliac vein, left common femoral vein, and left femoral vein (Fig. 1). Given the findings, Interventional Radiology (IR) was consulted for endovascular thrombectomy. The patient was brought to IR suite and placed in prone position. Following the standard prep, through right popliteal vein access, a FlowTriever Catheter (Inari Medical, Irvine, California) with nitinol mesh disks was placed in the proximal IVC as a distal embolic protection device. Then, venous access for thrombectomy was achieved through the left popliteal vein. An ascending venogram confirmed that the left femoral, common femoral, external iliac and common iliac veins were fully occluded (Fig. 2A) with clot extending cephalad into the distal IVC. Mechanical thrombectomy was performed using ClotTriever System (Inari Medical, Irvine, California) with successful removal of the clot (Fig. 2B), revealing a mixture of chronic and subacute thrombus.
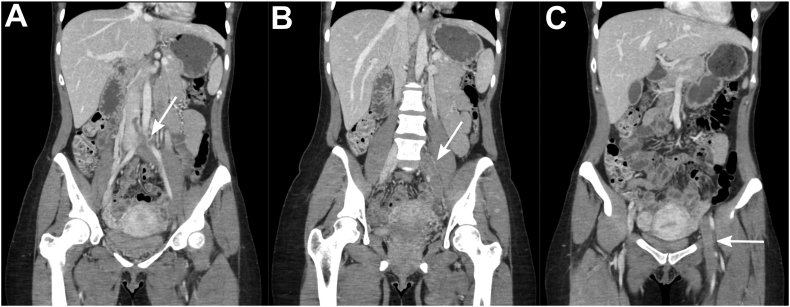
Fig. 1.
CT Venogram showing extent of thrombus. A. Arrow shows thrombus from left common iliac vein extending to the IVC. B. Arrow shows thrombus in the left common iliac and left external iliac veins. C. Arrow shows thrombus in the left common femoral vein and femoral vein.
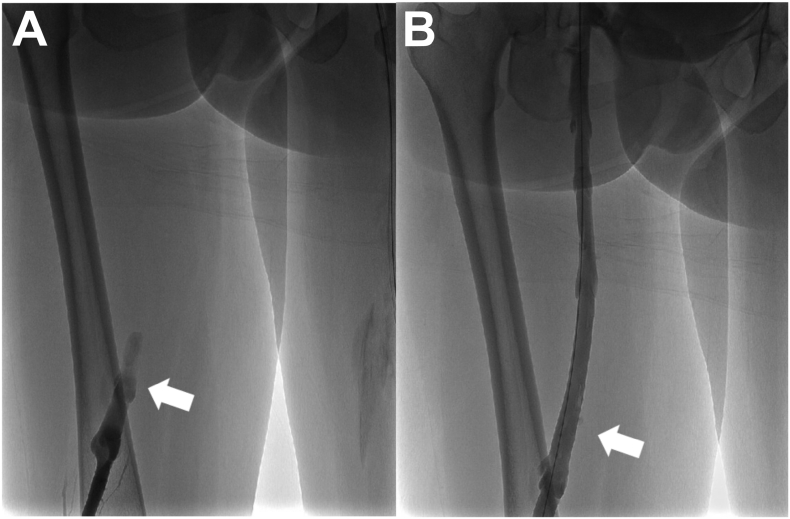
Fig. 2.
Ascending venogram with contrast of the left internal iliac vein. A. Arrow shows total occlusion of the left internal iliac vein in venogram performed prior to thrombectomy. B. Post-thrombectomy venogram showing complete resolution of the occlusion. Arrowhead denotes the approximate location of the arrowhead in panel A.
Subsequent intra-operative venogram revealed complete resolution of the thrombus but a focal stenosis at the origin of the left common iliac vein consistent with extravascular compression (Fig. 3). Intravascular ultrasound (IVUS) was used to assess the severity and etiology of stenosis. IVUS revealed high-grade left common iliac vein stenosis due to compression from an overlying right common iliac artery, consistent with a May-Thurner lesion (Fig. 4). Chronic compression predisposes these lesions to re-thrombosis after thrombectomy despite anticoagulation therapy [9]. Thus, transluminal angioplasty and stenting of the left common iliac vein were performed to restore the vessel lumen.
Fig. 3.
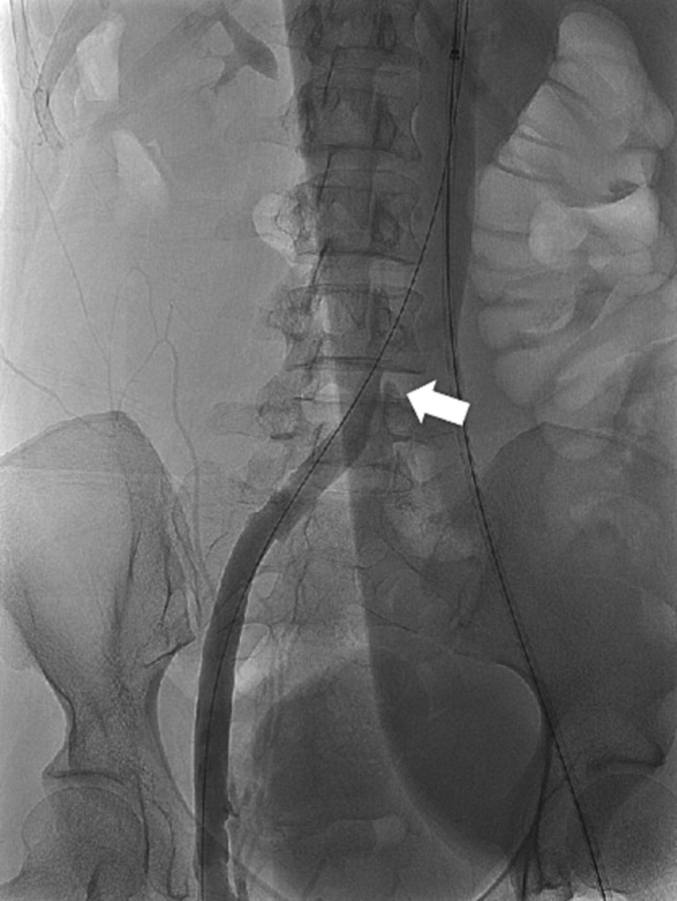
Post-thrombectomy ascending venogram with contrast showing focal area of stenosis (arrowhead) at the origin of the left IVC consistent with a May-Thurner lesion.
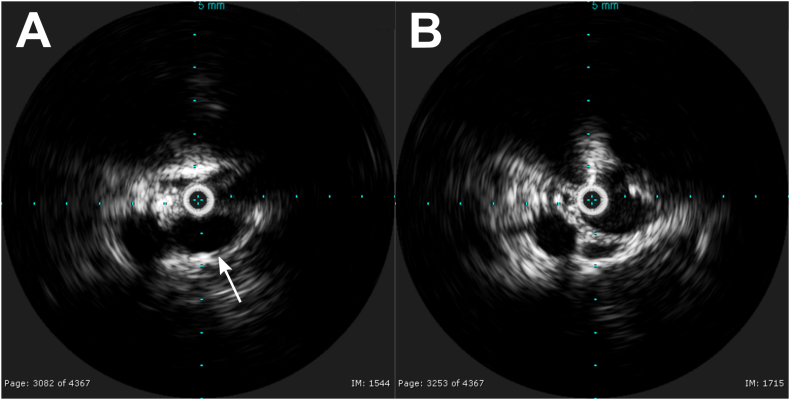
Fig. 4.
Intravenous ultrasound (IVUS) showing left common iliac vein compression. A. IVUS within the left common iliac vein showing no appreciable lumen of the vein due to complete compression by right common iliac artery positioned directly anterior (arrow). Note that the patient was prone, so the top of image is posterior, and bottom is anterior. B. IVUS within the left common iliac vein at an area adjacent to the compression showing a normal vein lumen.
Collectively these measures successfully restored venous blood flow. Postprocedural venography and intravascular ultrasonography demonstrated complete resolution of the lesion and brisk flow of contrast throughout the left leg (Fig. 5).
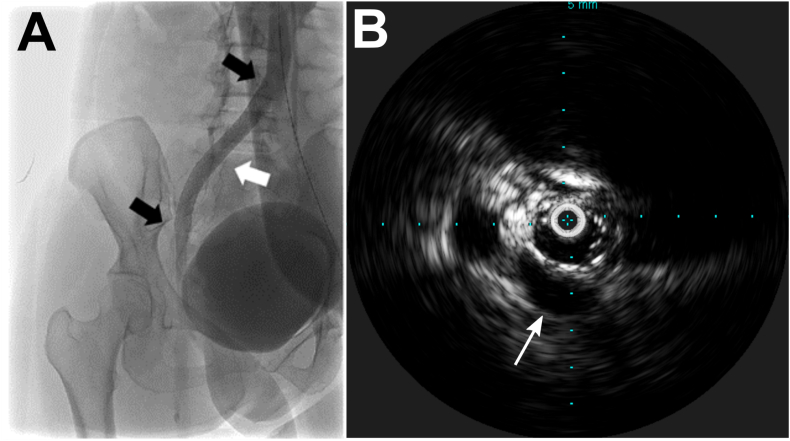
Fig. 5.
Resolution of left common iliac vein compression after angioplasty and stenting. A. Ascending venogram with contrast showing resolution of the stenosis. White arrowhead denotes approximate location of prior stenosis noted in Fig. 3. Black arrows show the proximal and distal extent of the stent. B. IVUS within the stented left common iliac vein showing that the compression is resolved. Arrow shows right common iliac artery.
Symptomatic MTS is thought to be uncommon relative to the prevalence of the underlying lesion [8]. Thus, additional radiographic workup for etiologies of hypercoagulable states outside of the patient's OCP use were warranted. Notably, CT imaging at the patient's initial presentation had incidentally found a small lesion within the pancreatic tail. Given the context of these findings, MRI of the abdomen with contrast was performed. It revealed a small pancreatic neoplasm.
The patient's swelling and pain resolved rapidly after the procedure, and she was discharged on anticoagulation and antiplatelet therapy as well as compression stockings with discontinuation of the OCPs. Venous duplex ultrasound at 6-month follow-up demonstrated no re-thrombosis and a patent left iliac vein stent (Fig. 6). Follow-up pancreatic lesion biopsy confirmed a low-grade malignant neuroendocrine tumor of the pancreatic tail, and care was transferred to an outside institution for oncological follow-up and management.
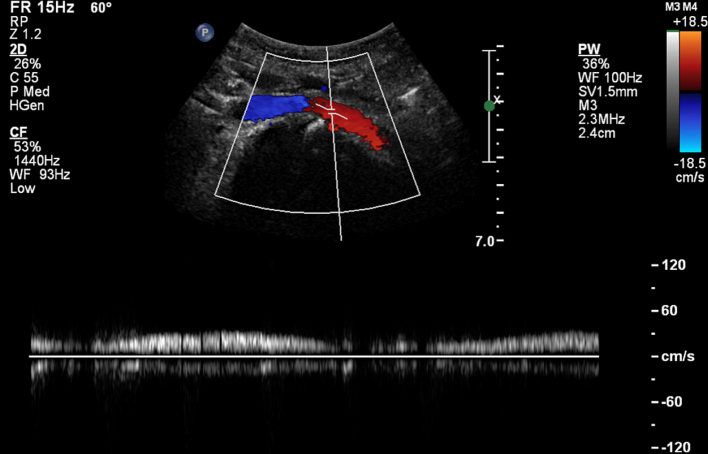
Fig. 6.
Duplex ultrasound of the left common iliac vein at the level of the placed stent at 6-months follow-up. Colors show blood flow from the left common iliac vein (red) towards the IVC (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
This case presents an adult female with extensive left lower extremity thrombosis in the setting of a May-Thurner lesion, oral contraceptive use, and previously undiagnosed malignancy. Previous reviews and case reports have summarized the general features of MTS [2,8,10,11]. Therefore, we focus the discussion herein on the unique features of this case: the severity of the underlying lesion and malignancy as a predisposing factor for MTS.
In the past decade, intravascular ultrasound has been widely adopted for use in diagnosing MTS [5,12]. This approach allows both diagnosis and simultaneous evaluation of the severity of the iliac vein stenosis. Interestingly, recent research found that the severity of MTS stenosis as indicated by IVUS is not an accurate predictor of initial clinical presentation nor initial success of endovascular stenting [5]. Consistent with this work, we were able to achieve full resolution of the compression with angioplasty and stenting, despite a severe iliac vein compression. In contrast, stenosis is predictive of symptom recurrence. Patients with >90 % stenosis, such as the one presented here, have an increased recurrence of pain, swelling, and worse venous clinical severity scores at four-year follow-up [5]. Patients with severe stenosis should be counseled of this risk.
Our choice of angioplasty and stenting after initial thrombectomy is consistent with the recommended initial management of MTS and has a technical success rate of over 95 % [2,13,14]. Indeed, a review of 119 patients with MTS found that 96 % of patients had maintained patency of the iliac vein 1-year post endovascular intervention [15]. However, it is important to consider that technical success of endovascular therapy does not always equate to complete resolution of clinical symptoms [16]. Our choice of angioplasty and stenting was informed by evidence that May-Thurner lesions are stubbornly recurrent even in patients on anticoagulation. Evidence suggests reoccurrence rates of over 70 % in patients who undergo angioplasty without stent placement [9].
May-Thurner lesions are underdiagnosed and often asymptomatic. Cadaver studies suggest the lesion is present in 14–36 % of the general population [8]. A retrospective chart review found that over 27 % of a sample of 50 consecutive patients presenting to an emergency room had evidence of the lesion on abdominal CT but were asymptomatic [14]. One explanation of these findings is that the anatomic variant alone may only infrequently precipitate thrombosis in absence of a secondary insult. Consistent with this hypothesis, studies have found that many patients with MTS have an underlying hypercoagulable disorder [2,[18], [19], [20], [21], [22]]. For example, MTS has been associated with pregnancy, prolonged immobility, OCP use, and the postpartum period (Table 1). However, there are many cases of MTS with no known predisposing factors outside of the May-Thurner lesion itself [6,[14], [15], [16],[23], [24], [25]]; highlighting how the risk factors for MTS remain unclear.
Table 1.
Review of cases of May-Thurner Syndrome in the literature and associated predisposing factors.
| Patient age and sex | Suggested predisposing factors | Intervention | Citation |
|---|---|---|---|
| 27 yo female | Pregnancy | Therapeutic Heparin | Nakajima et al., 2012 [21] |
| 31 yo male | Trauma/prolonged immobility | Endovascular catheter-directed thrombolysis with balloon angioplasty and stent placement | Sinani et al., 2021 [22] |
| 7 patients: 16–24 yo female | Oral contraceptive pills | Endovascular thrombolysis and stent placement | Murphy et al., 2009 [20] |
| 3 patients: 20–28 yo female | Postpartum | Endovascular thrombolysis and stent placement | Zander et al., 2008 [18] |
| 10 patients: 22–52 yo female | 1 patient: metastatic adenocarcinoma 9 patients: No known coagulopathy reported |
Endovascular catheter-directed thrombolysis with balloon angioplasty and/or stent placement | Patel et al., 2000 [11] |
| 23 yo male | No known factors reported | Endovascular thrombectomy, thrombolysis, angioplasty, and stent placement | Hng et al., 2021 [23] |
| 60 yo female | No known factors reported | Endovascular balloon angioplasty and stent placement | Meng et al., 2020 [24] |
| 29 yo male | No known factors | Endovascular angioplasty and stent placement | Seely et al., 2022 [25] |
| 57 yo male | No known factors reported | Endovascular angioplasty, maceration, and stent placement | Shafari and Farsad, 2018 [7] |
| 39 yo male | No known factors reported | Surgical angioplasty and thrombectomy | Steinberg and Jacocks, 1993 [6] |
| 8 patients: 52–78 yo | No known factors reported | Endovascular balloon angioplasty, thrombolysis, and stenting | Igari et a., 2014 [14] |
| 10 yo male | No known factors reported | Endovascular balloon angioplasty and stent placement | Oguzkurt et al., 2006 [16] |
| 18 yo female | No known coagulopathy reported | Endovascular catheter-directed thrombolysis and stent placement | Moudgill et al., 2009 [15] |
The patient presented here had two hypercoagulability risk factors that likely both contributed to her MTS. First, she had recently begun oral OCPs. Previous studies have linked OCPs and development of MTS [2,8,17]. The development of symptomatic DVT occurs rapidly: within 2–10 weeks after initiation of OCPs [17]. Consistent with this, our patient was on OCPs for approximately 16 weeks by the time of her initial presentation, suggesting that OCPs were a contributing factor.
Second, the patient had underlying pancreatic cancer that likely predisposed her to developing MTS. The patient's only constitutional symptom was occasional heavy night-sweats, and the pancreatic lesion was an incidental finding on a CT in the ED at her initial presentation. Her extensive thrombus with mixed chronic and subacute clot put this pancreatic lesion in context and prompted subsequent work-up that identified the malignancy. The work presented here has been reported in line with the SCARE criteria for surgical case reports [26].
4. Conclusion
In summary, this case showcases endovascular management of an extensive clot with severe underlying anatomical compression of the left common iliac vein in the setting of multiple predisposing factors for MTS. While the predisposing factors for MTS remain unclear, hypercoagulable states including OCP use, pregnancy, prolonged immobilization and malignancy have been associated with the condition. Future research should investigate the incidence of venous thrombosis amongst patients with May-Thurner lesions versus those without the anatomic predisposition and attempt to elucidate contributing factors. In addition, the field would benefit from large studies investigating the relationship between the degree of stenosis, symptom severity, and long-term patency following endovascular management.
Ethical approval
Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. Per institutional policy, ethics approval is not required for appropriately de-identified case reports.
Funding
This study was not supported by any funding.
Author contribution
CJM: manuscript writer/editor. EI: manuscript writer/editor. AS: manuscript writer/editor Radiology Resident. FK: manuscript writer/editor Interventional Radiology Attending.
Guarantor
Dr. Francis Kang, MD.
Dr. Camden MacDowell, PhD.
Research registration number
NA.
Conflict of interest statement
The authors have no conflict of interest.
Acknowledgements
None.
Contributor Information
Camden J. MacDowell, Email: cjm367@rwjms.rutgers.edu.
Emma Idzikowski, Email: eci6@rwjms.rutgers.edu.
References
- 1.May R., Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 2.Mousa A.Y., AbuRahma A.F. May-Thurner syndrome: update and review. Ann. Vasc. Surg. 2013;27:984–995. doi: 10.1016/j.avsg.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Hager E.S., Tahara R., Dillavou E., Al-Khoury G., Yuo T., Rhee R., Marone L., Makaroun M., Chaer R.A. Long-term outcomes of endovascular intervention for May-Thurner syndrome. J. Vasc. Surg. 2012;55:304. doi: 10.1016/j.jvs.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Burke R.M., Rayan S.S., Kasirajan K., Chaikof E.L., Milner R. Unusual case of right-sided May-Thurner syndrome and review of its management. Vascular. 2006;14:47–50. doi: 10.2310/6670.2006.00012. [DOI] [PubMed] [Google Scholar]
- 5.Jayaraj A., Buck W., Knight A., Johns B., Raju S. Impact of degree of stenosis in May-Thurner syndrome on iliac vein stenting. J. Vasc. Surg. Venous Lymphat. Disord. 2019;7:195–202. doi: 10.1016/j.jvsv.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg J.B., Jacocks M.A. May-Thurner syndrome: a previously unreported variant. Ann. Vasc. Surg. 1993;7:577–581. doi: 10.1007/BF02000154. [DOI] [PubMed] [Google Scholar]
- 7.Sharafi S., Farsad K. Variant May-Thurner syndrome: compression of the left common iliac vein by the ipsilateral internal iliac artery. Radiol. Case Rep. 2018;13:419–423. doi: 10.1016/j.radcr.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbin M.M., Lutsey P.L. May-Thurner syndrome: history of understanding and need for defining population prevalence. J. Thromb. Haemost. 2020;18:534–542. doi: 10.1111/jth.14707. [DOI] [PubMed] [Google Scholar]
- 9.Mickley V., Schwagierek R., Rilinger N., Görich J., Sunder-Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J. Vasc. Surg. 1998;28:492–497. doi: 10.1016/s0741-5214(98)70135-1. [DOI] [PubMed] [Google Scholar]
- 10.Speranza G., Sadek M., Jacobowitz G. Common iliac vein stenting for May-Thurner syndrome and subsequent pregnancy. J. Vasc. Surg. Venous Lymphat. Disord. 2022;10:348–352. doi: 10.1016/j.jvsv.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Patel N.H., Stookey K.R., Ketcham D.B., Cragg A.H. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J. Vasc. Interv. Radiol. 2000;11:1297–1302. doi: 10.1016/s1051-0443(07)61304-9. [DOI] [PubMed] [Google Scholar]
- 12.Majdalany B.S., Khaja M.S., Williams D.M. Intravascular ultrasound-guided intervention for May–Thurner syndrome. Semin. Intervent Radiol. 2017;34:201–207. doi: 10.1055/s-0037-1602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J., Liu Y., Zhou W. Mid-and long-term efficacy of endovascular-based procedures for Cockett syndrome. Sci. Rep. 2018;8:12145. doi: 10.1038/s41598-018-29756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igari K., Kudo T., Toyofuku T., Jibiki M., Inoue Y. Surgical thrombectomy and simultaneous stenting for deep venous thrombosis caused by iliac vein compression syndrome (May-Thurner syndrome) Ann. Thorac. Cardiovasc. Surg. 2014;20:995–1000. doi: 10.5761/atcs.oa.13-00213. [DOI] [PubMed] [Google Scholar]
- 15.Moudgill N., Hager E., Gonsalves C., Larson R., Lombardi J., DiMuzio P. May-Thurner syndrome: case report and review of the literature involving modern endovascular therapy. Vascular. 2009;17:330–335. doi: 10.2310/6670.2009.00027. [DOI] [PubMed] [Google Scholar]
- 16.Oguzkurt L., Tercan F., Sener M. Successful endovascular treatment of iliac vein compression (May-Thurner) syndrome in a pediatric patient. Cardiovasc. Intervent. Radiol. 2006;29:446–449. doi: 10.1007/s00270-004-0247-6. [DOI] [PubMed] [Google Scholar]
- 17.Kibbe M.R., Ujiki M., Goodwin A.L., Eskandari M., Yao J., Matsumura J. Iliac vein compression in an asymptomatic patient population. J. Vasc. Surg. 2004;39:937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Zander K.D., Staat B., Galan H. May-Thurner syndrome resulting in acute iliofemoral deep vein thrombosis in the postpartum period. Obstet. Gynecol. 2008;111:565–569. doi: 10.1097/01.AOG.0000299875.19865.4c. [DOI] [PubMed] [Google Scholar]
- 19.Wax J.R., Pinette M.G., Rausch D., Cartin A. May-Thurner syndrome complicating pregnancy: a report of four cases. J. Reprod. Med. 2014;59:333–336. [PubMed] [Google Scholar]
- 20.Murphy E.H., Davis C.M., Journeycake J.M., DeMuth R.P., Arko F.R. Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May-Thurner Syndrome. J. Vasc. Surg. 2009;49:697–703. doi: 10.1016/j.jvs.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima Y., Masaoka N., Tsuzuki Y., Morooka M., Sakai M. May-Thurner syndrome resulting in acute iliofemoral deep vein thrombosis during the second trimester of pregnancy. J. Obstet. Gynaecol. Res. 2012;38:1106–1110. doi: 10.1111/j.1447-0756.2011.01840.x. [DOI] [PubMed] [Google Scholar]
- 22.A. Al Sinani, W. Al Saadi, S. Al Harthi, M. Al Hajriy, May-Thurner syndrome: a case report and a concise review, Cureus 13 (n.d.) e16256. 10.7759/cureus.16256. [DOI] [PMC free article] [PubMed]
- 23.Hng J., Su S., Atkinson N. May-Thurner syndrome, a diagnosis to consider in young males with no risk factors: a case report and review of the literature. J Med Case Reports. 2021;15:141. doi: 10.1186/s13256-021-02730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Y., Qin H., Ma Q., Zhang J., Zhang B., Pang H., Yin Q., Tian H. Deep vein thrombosis due to May-Thurner syndrome: a case report. BMC Cardiovasc. Disord. 2020;20:233. doi: 10.1186/s12872-020-01515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seely K.D., Arreola H.J., Paul L.K., Higgs J.A., Brooks B., Anderson R.C. Seizures, deep vein thrombosis, and pulmonary emboli in a severe case of May-Thurner syndrome: a case report. J Med Case Reports. 2022;16:411. doi: 10.1186/s13256-022-03639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]