Summary
Regular physical exercise is beneficial to the body. Acute exercise causes oxidant stress in many tissues including the liver by creating an unbalanced status between oxidant and antioxidant levels. Analgesic drugs are commonly consumed to reduce the pain after exercise. Acetaminophen (APAP), commonly used as an over-the-counter analgesic, can cause hepatotoxicity. The aim of this study was to investigate the effect and underlying mechanisms of APAP at subtoxic dose, which is given after the acute and exhaustive exercise on the rat livers. Male Wistar rats weighing 200–250 g were divided into 6 groups each consisting of 7 rats/group; Control, APAP (250 mg/kg, ip), Acute Exercise (AEx), Acute Exhaustive Exercise (AEEx), Acute Exercise and APAP (AEx+APAP) and Acute Exhaustive Exercise and APAP (AEEx+APAP) groups. Rats were exercised at moderate intensity or exhaustive on the treadmill and then received APAP. Tissue MDA levels were significantly increased in AEEx, AEx+APAP and AEEx+APAP groups compared with the control. There was no significant difference in GSH levels between groups. Tissue Sirtuin1 (Sirt1) levels of APAP, AEx and AEEx groups were significantly less than control. There was no significant difference between groups in VEGF levels. Liver damage score was significantly higher in all groups compared with control group. As a result, this study shows that subtoxic dose of APAP treatment alone or in combination with acute or exhaustive treadmill exercise can cause oxidative liver damage by affecting Sirt1 levels and without affecting VEGF levels.
Keywords: Exercise, Acetaminophen, Oxidative stress, Sirtuin1, Liver
Introduction
Physical activity is seen and recommended by scientists throughout life as an important component of a healthy lifestyle [1]. Oxidative stress is the result of an imbalance between the production of reactive oxygen species (ROS) and the ability to detoxify reactive intermediates or to repair the resulting damage by an adequate antioxidant defense. Oxidative stress may lead to damage of all cellular components including proteins, lipids, carbohydrates, and nucleic acids. Oxidative stress is recognized to be involved in many physiological conditions (e.g. aging and exercise) and diseases (including inflammation, cardiovascular and neurodegenerative diseases, and cancer) [2]. In particular, the effect of exercise on redox balance is extremely complex, depending on age, sex, and training level, as well as intensity and duration of exercise. Although regular moderate training appears beneficial for oxidative stress and health, acute and strenuous bouts of aerobic and anaerobic exercise can induce ROS overproduction [3].
Glutathione (gamma-glutamylcysteinylglycine) is one of the major antioxidants in the body. Glutathione normally protects against arylation and/or oxidation. Glutathione in the body is reduced by prolonged physical exercise [4]. It is concluded that exhaustive exercise can impose severe oxidative stress on skeletal muscle and that glutathione systems as well as antioxidant enzymes are important in coping with free radical-mediated muscle injury [5].
Acetaminophen (APAP) is an over-the-counter drug used widely across the world with potent analgesic and antipyretic properties [6]. APAP is safe and effective at recommended doses, but overdose of APAP may lead to liver injury progressing to acute liver failure (ALF) and death. At therapeutic doses, APAP is inactivated in the liver as sulfate and glucuronide conjugates. However, intermediate metabolites with high toxicity (N-acetyl-p-benzoquinone-imine=NAPQI) are formed with cytochrome P450 enzymes. These metabolites are reduced by glutathione in the liver and eliminated in the urine as cysteine conjugates. Overdose of paracetamol increases the formation of these toxic metabolites and leads to rapid depletion of limited glutathione stores in the liver. NAPQI binds irreversibly with macromolecules that cause necrosis in hepatocytes and causes liver damage [7,8]. APAP can cause liver damage even at subtoxic doses [7]. APAP-induced hepatotoxicity is the principal cause of ALF and an important persistent public health concern in many countries [9]. However, the mechanism of this liver injury is not fully understood. The generation of ROS, nitric oxide and lipid peroxides are assumed to be the major factors involved in this liver injury [10].
Sirtuin 1 (Sirt1), a member of nicotinamide adenine dinucleotide (NAD+) dependence protein deacetylase, has an important role in anti-apoptosis, repair of DNA damage, energy metabolism and mitochondrial function protection [11]. Sirt1 itself has multiple biological activities including gene silencing, apoptosis, stress resistance, regulation of cell proliferation, aging, and inflammation. It also acts as the molecular sensor of nutritional status and protects against oxidative stress [12]. It has been shown in a study that; after acute APAP treatment protein levels of Sirt1 in the liver of humans and mice are decreased and also that moderate Sirt1 overexpression in mice protects against inflammation and oxidative stress during APAP hepatoxicity [13]. Decreased Sirt1 expression and increased oxidative stress play a role in APAP-induced liver injury. However, the role of Sirt1 in APAP administration with acute or exhaustive exercise is unclear. In future studies, pharmacological approaches or supplements that modulate Sirt1 expression may be effective in reducing liver damage. Therefore, the role of Sirt1 was investigated in this study.
Acute exercise significantly increases the gene expression of growth factors believed to be responsible for exercise-induced angiogenesis, with the greatest exercise induced increase observed in vascular endothelial growth factor (VEGF) [14]. VEGF is a highly specific mitogen for vascular endothelial cells. VEGF induces endothelial cell proliferation, promotes cell migration, and inhibits apoptosis. In vivo VEGF has been shown to induce angiogenesis as well as permeabilization of blood vessels, and plays a central role in the regulation of vasculogenesis [15]. In a study conducted to examine the role of VEGF in APAP-induced hepatotoxicity it has been found that endogenous VEGF is important in the recovery stages of APAP toxicity and neutralization of VEGF slows the recovery process and is associated with a reduction of proteins previously implicated in the process of angiogenesis [16]. It has been reported that in drug-induced liver toxicity, hepatic VEGF levels decrease or do not change in the early period but increase in the later stages [16,17]. Due to its anti-apoptotic and anti-fibrotic effects, increased VEGF during the recovery period may be effective in reducing liver damage. Studies on the effects of APAP administration on VEGF after acute exercise in the acute period has not been found in the literature. Therefore, the levels of VEGF were investigated in this study.
Studies on if acute exercise causes oxidant stress on the liver or not are few and the results of these studies are conflicting. The effects of APAP on this phenomenon are unknown. Research on the effects of varying doses of APAP on the oxidative stress on the liver after varying intensity levels of exercise can be helpful for people both healthy and with hepatic damage to make better choices in exercise programs and the use of analgesics after exercise. This study aims to investigate the effects of subtoxic doses of APAP given after acute and exhausting exercise on the oxidative stress and the role of Sirt1 and VEGF in the rat liver.
Methods
Animals
The study was carried out on male Wistar rats weighing 200–250 g. They were housed in polycarbonate cages with food and water ad libitum. All experimental protocols were approved by the Animal Research Ethics Committee of the Dokuz Eylul University Medical Faculty, Turkey (approval number: 10/2008). The rats were maintained in a constant 12-h light/12-h dark cycle at constant room temperature (22±1 °C) and humidity (60 %). The rats were divided into 6 groups each consisting of 7 rats/group:
Group 1: Control group (C).
Group 2: Acetaminophen group (APAP) was injected with a single dose of APAP 250 mg/kg, i.p. (Bristol-Myers Squibb, Princeton, New Jersey, USA).
Group 3: Acute Exercise group (AEx). Animals were run on the treadmill at 10 m/min, 5 % grade for one hour.
Group 4: Acute Exhaustive Exercise group (AEEx). Animals were run on the treadmill at 25 m/min until exhaustion.
Group 5: Acute Exercise and APAP group (AEx+APAP).
Group 6: Acute Exhaustive Exercise and APAP group (AEEx+APAP).
The rats except control and APAP groups performed treadmill running that rats were run on a motor-driven rodent treadmill. The rats in the exercise groups were familiarized by treadmill running for five days for 10 min/day at 10 m/min, 0 % grade. Electric shocks gradually were given to motivate the animals to run. The backward-moving rats were repositioned by the researchers. Acute and exhaustive exercises were applied 2 days after the familiarization period. Exhaustion was defined as touching the electrified grid behind the treadmill five times in 2 min [18]. AEx+APAP and AEEx+APAP groups received a dose of 250 mg/kg APAP immediately after exercise and the exhaustion test [7]. Control and APAP groups were kept only in the exercise room.
4 h after acute exercise and exhaustion test, the rats in each group were sacrificed under mild ether anesthesia. The livers were removed and placed on an ice-cold surface. Liver tissue was divided into two parts. The first piece was processed for histological examination. The second part was homogenized for tissue biochemical estimations.
Biochemical evaluation
The liver tissue samples were weighed after cleaning with physiological saline solution and homogenized (10 %, w/v) with phosphate buffer (PBS, pH 7.4) using an ultrasonic homogenizer (Bandelin Sonopuls, Germany). The homogenates were centrifuged at 10000× g for 20 min at 4 °C. Tissue MDA levels were measured spectrophotometrically using the Bioxytech MDA-586 (Oxis International, USA) commercial kit. The method of the kit is based on the reaction of MDA with a chromogenic reagent at 45 °C. MDA values were determined from the standard curve by measuring the absorbance at 586 nm. Results were expressed as μM/mg protein. GSH levels in tissues were measured by spectrophotometer (T80 PG, instruments, UK) using GSH-420 commercial kit (Oxis International, USA). The method of the kit is based on the formation of chromophoric thione. The oxidizing glutathione is converted into a reduced form by adding reducing agent to the buffer-mixed supernatant. Chromophoric thione was formed by adding chromogen and increasing the pH value. GSH concentration was determined by measuring the absorbance at 420 nm. Results were expressed as μM/mg protein. For the measurement of tissue protein levels, Pierce BCA Protein Assay Kit (23227, Thermo Scientific, Illinois, USA) was used.
Sirt1 and VEGF levels were measured using a plate reader (BioTek ELx800, US) with commercial kits (Cat. No: E-EL-R1102, Elabscience, USA and 201-11-0660, Sunred Biotechnology, China, respectively) as recommended by the manufacturer. Results were expressed as ng/mg protein and pg/mg protein, respectively. Tissue samples were weighed and homogenized in PBS, and then centrifuged for 20 min at 5000× g at 4 °C to isolate the supernatant. To determine Sirt1 levels, 100 μl sample was added into a 96-well plate and incubated at 37 °C for 90 min. Then, 100 μl of biotin was added and incubated at 37 °C for 1 h, followed by addition of HRP and incubation for 30 min at 37 °C, and then dark incubation with substrate reagent at 37 °C for 15 min. The optical density was measured at 450 nm and the concentration of Sirt1 was calculated by the corresponding standard curve. To determine VEGF levels, 50 μl enzyme-labeled reagents were added to the 96-well-plate, except the blank control and incubated at 37 °C for 1 h. Then, substrates A and B (50 μl each) were added followed by dark incubation with substrate reagent at 37 °C for 10 min. The optical density of VEGF was measured at 450 nm and the concentration was calculated using standard curve regression method.
Liver function markers were assayed by measuring spectrophotometrically the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using original kits on the Beckman Coulter AU5800 analyzer (USA).
Histological evaluation
The liver samples were fixed in 10 % formalin solution at room temperature for 48 h, and then passed through an increasing series of alcohol. After the tissues were exposed to three exchanges of xylol for transparency, they were embedded in paraffin. The sections of 5 μm thickness were taken from the paraffin blocks with a microtome (Thermo Finesse ME+, Thermo Scientific). Sections were stained with hematoxylin-eosin (H&E) and Masson trichrome to examine under a light microscope. Liver samples were analyzed semi-quantitatively and graded as; no damage (0; −), mild (1; +), moderate (2; ++), and severe (3; +++). Histological score was evaluated as congestion, mononuclear cell infiltration, pycnotic nucleus, and sinusoidal dilatation [7].
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). Data analyses were carried out using the SPSS 27.0 (IBM Inc., IL, USA) for Windows software. Differences between the groups were analyzed using the one-way analysis of variance (ANOVA) post hoc LSD test. Kruskal Wallis test was used for data that did not show normally distribution. The values of p<0.05 were considered statistically significant.
Results
Biochemical evaluation
The mean MDA levels of all groups are shown in Table 1. There was a significant difference between MDA values of control, AEEx, AEx+APAP and AEEx+APAP groups (F(5,32)=15.96, p<0.001). MDA values of AEEx, AEx+APAP and AEEx+APAP groups were significantly increased compared with the control group (p<0.001). MDA values of AEEx group were significantly increased compared with the APAP group (p<0.001). There was no significant difference between control and APAP group (p>0.05).
Table 1.
MDA and GSH levels and damage score of rat livers.
| Groups | MDA (Mean ± SEM) (μM/mg protein) | GSH (Mean ± SEM) (μM/mg protein) | Damage score (Mean ± SEM) |
|---|---|---|---|
| Control | 1.07 ± 0.06 | 225.41 ± 23.0 | 1.4 ± 0.24 |
| APAP | 0.83 ± 0.08 | 226.61 ± 26.56 | 3.2 ± 0.37* |
| AEx | 0.88 ± 0.18 | 217.14 ± 22.20 | 2.8 ± 0.37 |
| AEEx | 1.62 ± 0.07* | 240.04 ± 59.83 | 3.4 ± 0.24* |
| AEx+APAP | 1.68 ± 0.11* | 295.11 ± 23.96 | 3.4 ± 0.24* |
| AEEx+APAP | 1.70 ± 0.04* | 228.66 ± 26.57 | 3.6 ± 0.24* |
| p (AEEx vs. control) | <0.001 | NS | <0.001 |
| p (AEx+APAP vs. control) | <0.001 | NS | <0.001 |
| p (AEEx+APAP vs. control) | <0.001 | NS | <0.001 |
| p (AEEx vs. APAP) | <0.001 | NS | NS |
| p (APAP vs. control) | NS | NS | <0.001 |
The mean GSH levels of all groups are shown in Table 1. There was no significant difference between all groups (p>0.05).
The mean Sirt1 and VEGF levels of all groups are shown in Table 2. There was a significant difference between Sirt1values of groups (F(5,24)=4.52, p=0.005). Sirt1 levels of APAP, AEx and AEEx groups were significantly less than control group (p=0.002, p=0.004 and p<0.001, respectively). Sirt1 levels of AEEx group were significantly less than AEx+APAP and AEEx+APAP groups (p=0.033 and p=0.017, respectively). There was no significant difference between groups in VEGF levels (p>0.05).
Table 2.
Sirt 1 and VEGF levels of rat livers (mean ± SEM).
| Groups | Sirt1 (ng/mg protein) | VEGF (pg/mg protein) |
|---|---|---|
| Control | 11.48 ± 1.41 | 403.84 ± 125.27 |
| APAP | 7.00 ± 0.49* | 196.29 ± 24.45 |
| AEx | 7.31 ± 0.55* | 199.73 ± 49.45 |
| AEEx | 6.18 ± 0.66* | 224.15 ± 31.67 |
| AEx+APAP | 9.14 ± 0.49 | 235.90 ± 6.19 |
| AEEx+APAP | 9.55 ± 1.37 | 252.70 ± 46.04 |
| p (APAP vs. control) | 0.002 | NS |
| p (AEx vs. control) | 0.004 | NS |
| p (AEEx vs. control) | <0.001 | NS |
| p (AEx+APAP vs. AEEx) | 0.033 | NS |
| p (AEEx+APAP vs. AEEx) | 0.017 | NS |
The mean ALT and AST levels of all groups are shown in Table 3. There was no significant difference between groups in ALT levels (p>0.05). There was a significant difference between AST levels of groups (F(5,32)=4.09, p=0.005). AST levels of APAP, AEx, AEEx and AEEx+APAP groups were significantly higher than control group (p=0.019, p=0.009, p<0.001 and p<0.001, respectively).
Table 3.
ALT and AST levels in the serum of rats (mean ± SEM).
| Groups | ALT (U/l) | AST (U/l) |
|---|---|---|
| Control | 42.14 ± 1.23 | 123.85 ± 5.61 |
| APAP | 43.40 ± 2.12 | 209.50 ± 29.99* |
| AEx | 43.85 ± 2.33 | 220.00 ± 32.54* |
| AEEx | 40.02 ± 2.65 | 251.71 ± 38.83* |
| AEx+APAP | 34.11 ± 2.05 | 181.83 ± 11.83 |
| AEEx+APAP | 40.10 ± 4.14 | 251.20 ± 27.05* |
| p (APAP vs. control) | NS | 0.019 |
| p (AEx vs. control) | NS | 0.009 |
| p (AEEx vs. control) | NS | <0.001 |
| p (AEEx+APAP vs. control) | NS | <0.001 |
Histological evaluation
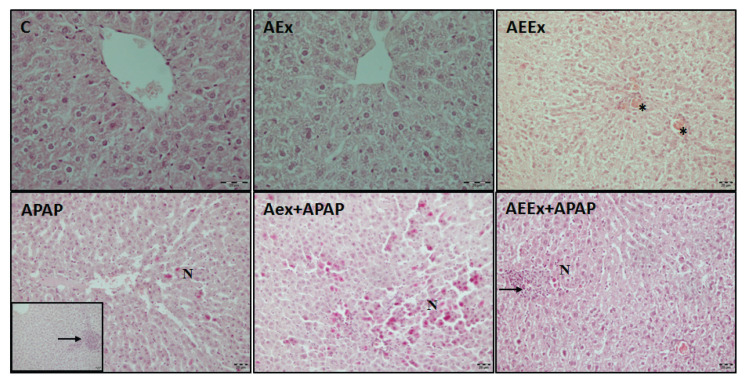
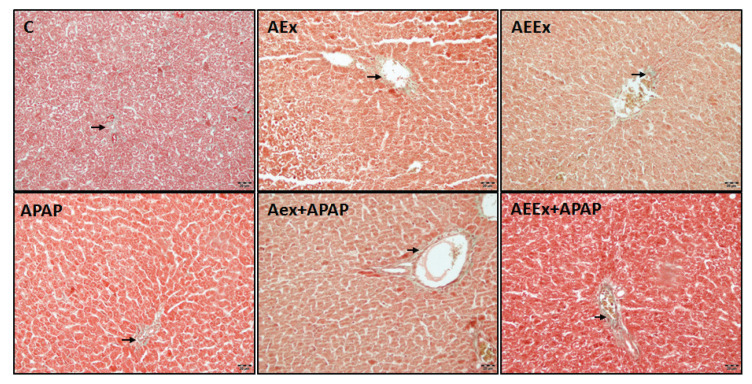
Liver images stained with H&E and Masson’s trichrome are presented in Figure 1 and Figure 2. In the sections of the control group, it was observed that the liver tissue had a normal histological appearance. It was observed that the liver tissues of the APAP, AEx and AEEx groups were affected compared to the control. In these groups, hepatocytes vacuolization, dilatation of sinusoids, congestion, focal inflammatory cell infiltration, hepatocytes leading to necrosis with pycnotic nuclei were observed. When the exercise groups were compared, no difference was observed. Liver damage score was significantly higher in all groups compared with control group (F(5,24)=7.64, p<0.001, Table 1). In the sections stained with Masson trichrome, there was no interstitial fibrosis in the control group, thin collagen fibers were observed around the central vein wall and sinusoidal wall. The distribution of collagen fibers in the APAP and exercise groups was similar to the control group, and no significant collagen deposition were observed.
Fig. 1.
Representative photomicrographs of H&E staining. Stars indicate congestion, black arrows; inflammatory cell infiltration, N; necrotic areas, bar=20 μm.
Fig. 2.
Representative photomicrographs of Masson trichrome staining. Arrows; collagen fibers, bar=20 μm.
Discussion
In this study, the effects of APAP on rat liver that underwent acute and exhaustive exercise were evaluated using biochemical and histological parameters. The findings of the study showed that exhaustive exercise and acute exercise combined with APAP lead the oxidative and histological changes in rat liver. Results showed that APAP and exhaustive exercise decreased liver Sirt1 level and no difference in VEGF levels. In previous studies, chronic exercise in diabetic or high-fat fed rats has been shown to increase Sirt1 levels in the liver, but there is no information about its mediating effects on exhaustive exercise-induced liver injury [19,20].
Oxidative stress plays a role in the pathogenesis of many diseases including liver injury. Oxidative stress is caused by the reaction of free radicals with biomolecules such as protein, DNA and lipids. Oxidation of lipids by free radicals is named as lipid peroxidation and leads to the formation of products such as malondialdehyde (MDA). MDA is considered one of the biomarkers of lipid peroxidation and is the most analyzed [21,22]. In our study, we found that MDA values in APAP combined with exercise groups increased significantly compared to control group. MDA, as a biomarker of oxidative damage due to APAP induced liver toxicity, has also been shown in previous studies [23,24]. Acute or exhaustive exercise can also cause oxidative stress in the liver [25,26]. In this study, subtoxic dose of APAP or acute exercise alone did not cause an increase in MDA, indicating that subtoxic dose of APAP causes oxidative stress when combined with acute or exhaustive exercise. Acute or exhaustive exercise potentiates the oxidative damaging effect of APAP. There is no information about effects of APAP when combined with acute or exhaustive exercise on oxidative liver injury.
Cells have enzymatic such as glutathione peroxidase (GPx) or superoxide dismutase (SOD) and non-enzymatic defense systems such as glutathione that buffer oxidative stress. Glutathione is converted to its oxidized form, GSSG, in the presence of free radicals [27]. In the present study, endogenous anti-oxidant parameter, GSH levels in the liver of all groups remained unchanged. Bouhlali et al. and Ding et al. reported that paracetamol-induced hepatotoxicity in rats decreased GSH content in the liver [28,29]. Similarly, exhaustive exercise reduces GSH levels in the liver by increasing free radical formation [30]. Since APAP applied in toxic doses cannot be detoxified, it causes a decrease in the GSH level in the liver. APAP administered at subtoxic doses may be rapidly metabolized in the liver, leading to maintenance of GSH levels.
Liver dysfunction may present with abnormalities in various biochemical markers. Drugs that cause hepatotoxicity, such as APAP, can alter the serum levels of various markers by impairing liver functions. This study showed that serum levels of AST were elevated after exposure to APAP and acute or exhaustive exercise. There are studies showing that liver damage due to APAP is associated with elevations in serum levels of ALT and AST [8,31]. On the other hand, it has been reported that there is an increase in AST levels in the acute period, but no change in ALT levels [32]. Biochemical markers may vary depending on the animal species used and the dose of APAP administered.
The present study suggests that APAP and acute or exhaustive exercise induces histological alterations in rat livers. Although there is no significant difference in the levels of biochemical markers when APAP is administered alone, histological data show changes in liver tissue. Gokkaya et al. observed that congestion, vacuolization and necrosis in rat livers of APAP group and the histopathological scores were significantly higher in the APAP group in comparison with control group [33]. Sinaga et al. and Kuvandik et al. reported that inflammation and necrosis in rat livers of APAP treated group [31,34]. Exhaustive exercise can also cause liver damage. Degeneration and lymphocytic infiltration were detected in the livers of exhaustive exercised rats after four weeks of training [35]. The results of our study regarding histological changes are in accordance with previous studies. In the present study, there were no significantly difference in damage scores between all groups except control. The severity of the damage did not show differences in the liver of APAP alone or exercise groups.
Sirt1 has a role in many biological processes such as energy metabolism, apoptosis and inflammation [36]. In this study, Sirt1 levels were found to be significantly lower in the livers of rats given APAP and subjected to acute or exhaustive exercise compared to the control group. Previous studies have shown that Sirt1 levels decrease in the livers of APAP treated rats [37,38,39]. Our data demonstrated that reduced levels of hepatic Sirt1 is associated with increased levels of MDA. Sirt1 plays a protective role against oxidative stress. Sirt1 prevents oxidative stress by deacetylation of transcription factors such as Nrf2 (Nuclear factor erythroid 2-related factor 2) and NF-kB (nuclear factor kB) in the liver. Sirt1 deficiency in the liver increases the formation of free radicals and causes oxidative stress [40]. Sirt1 levels contain conflicting results in exercise-applied studies. In rats with chronic liver disease, long-term exercise training increased Sirt1 levels compared to the patient group [19]. Similarly, long-term treadmill exercise increased Sirt1 expression in the liver in aged rats compared to the non-exercised group [41]. On the other hand, treadmill exercise alone did not cause significant changes in Sirt1 expression in the liver in high-fat-fed diabetic rats [42].
VEGF is a member of the growth factors family. In this study, APAP alone or in combination with exercise was found to reduce VEGF levels, but not statistically significant. In experimental liver damage models, the expression of angiogenic growth factors such as VEGF increases [43], but there are conflicting data about VEGF levels in liver damage. While some studies reported that VEGF levels are reduced in liver damage, some studies reported that increased or not changed [17,44,45]. Time course of VEGF expression has been demonstrated in APAP-induced liver toxicity models in experimental animals [16,32]. There are no studies on the effects of APAP administration on liver VEGF after acute exercise in the literature.
In this study, liver necrosis was observed in the APAP and exercise groups. As a result of damage to cell membranes and necrosis, intracellular enzymes are released into the blood and inflammation develops. Thus, in parallel with the histologically demonstrated damage findings, an increase in AST levels was also observed. In addition, the increase in ROS induced by APAP and acute exhausting exercise may have also caused tissue damage, leading to histological findings described. The decrease in SIRT1 levels, which has antioxidant activity, is accompanied by damage findings together with an increase in MDA. Biochemical and histological findings in the acute period are similar when APAP is applied together with exercise. If evaluation is made in the later period, histological findings and biochemical markers may differ between groups depending on the tissue recovery process.
Conclusions
In conclusion, the results of this study show that subtoxic dose of APAP treatment alone or in combination with acute or exhaustive treadmill exercise can cause liver damage. APAP and exercise cause oxidative damage in the liver by affecting Sirt1 levels and without affecting VEGF levels. More studies are needed to better elucidate the underlying molecular mechanisms of APAP toxicity combined with exercise.
Acknowledgements
This study was supported by the Dokuz Eylul University Scientific Research Foundation (BAP) Grant No: 2009KBSAG058.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Donaldson LJ. Sport and exercise: the public health challenge. Br J Sports Med. 2000;34:409–410. doi: 10.1136/bjsm.34.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/A:1026032404105. [DOI] [PubMed] [Google Scholar]
- 4.Lew H, Quintanilha A. Effects of endurance training and exercise on tissue antioxidative capacity and acetaminophen detoxification. Eur J Drug Metab Pharmacokinet. 1991;16:59–68. doi: 10.1007/BF03189876. [DOI] [PubMed] [Google Scholar]
- 5.Ji LL, Fu R. Responses of glutathione system and antioxidant enzymes to exhaustive exercise and hydroperoxide. J Appl Physiol (1985) 1992;72:549–554. doi: 10.1152/jappl.1992.72.2.549. [DOI] [PubMed] [Google Scholar]
- 6.Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir D, Aksu I, Baykara B, Ates M, Sisman AR, Kiray M, Buyuk A, Uysal N. Effects of administration of subtoxic doses of acetaminophen on liver and blood levels of insulin-like growth factor-1 in rats. Toxicol Ind Health. 2016;32:39–46. doi: 10.1177/0748233713498439. [DOI] [PubMed] [Google Scholar]
- 8.Wojnarová L, Kutinová Canová N, Farghali H, Kučera T. Sirtuin 1 modulation in rat model of acetaminophen-induced hepatotoxicity. Physiol Res. 2015;64(Suppl 4):S477–S487. doi: 10.33549/physiolres.933205. [DOI] [PubMed] [Google Scholar]
- 9.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 10.Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJ, Chen TS. Diverse antioxidants protect against acetaminophen hepatotoxicity. J Biochem Mol Toxicol. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Zhang X, Hao J, Shen J, Fang J, Dong W, Wang D, Zhang X, Shui W, Luo Y, Lin L, Qiu Q, Liu B, Hu Z. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep. 2014;4:7456. doi: 10.1038/srep07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rada P, Pardo V, Mobasher MA, García-Martínez I, Ruiz L, González-Rodríguez Á, Sanchez-Ramos C, et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxid Redox Signal. 2018;28:1187–1208. doi: 10.1089/ars.2017.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin TP, Wagner PD. Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol (1985) 2001;90:1219–1226. doi: 10.1152/jappl.2001.90.4.1219. [DOI] [PubMed] [Google Scholar]
- 15.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. doi: 10.1096/fasebj.13.1.9. [DOI] [PubMed] [Google Scholar]
- 16.Donahower B, McCullough SS, Kurten R, Lamps LW, Simpson P, Hinson JA, James LP. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G102–G109. doi: 10.1152/ajpgi.00575.2005. [DOI] [PubMed] [Google Scholar]
- 17.Abdelbaset-Ismail A, Tharwat A, Ahmed AE, Khamis T, Abd El-Rahim IH, Alhag SK, Dowidar MF. Transplantation of adipose-derived mesenchymal stem cells ameliorates acute hepatic injury caused by nonsteroidal anti-inflammatory drug diclofenac sodium in female rats. Biomed Pharmacother. 2022;155:113805. doi: 10.1016/j.biopha.2022.113805. [DOI] [PubMed] [Google Scholar]
- 18.Acikgoz O, Aksu I, Topcu A, Kayatekin BM. Acute exhaustive exercise does not alter lipid peroxidation levels and antioxidant enzyme activities in rat hippocampus, prefrontal cortex and striatum. Neurosci Lett. 2006;406:148–151. doi: 10.1016/j.neulet.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 19.John A, Howarth FC, Raza H. Exercise alleviates diabetic complications by inhibiting oxidative stress-mediated signaling cascade and mitochondrial metabolic stress in GK diabetic rat tissues. Front Physiol. 2022;13:1052608. doi: 10.3389/fphys.2022.1052608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajighasem A, Farzanegi P, Mazaheri Z, Naghizadeh M, Salehi G. Effects of resveratrol, exercises and their combination on Farnesoid X receptor, Liver X receptor and Sirtuin 1 gene expression and apoptosis in the liver of elderly rats with nonalcoholic fatty liver. PeerJ. 2018;6:e5522. doi: 10.7717/peerj.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali FF, Rifaai RA. Preventive effect of omega-3 fatty acids in a rat model of stress-induced liver injury. J Cell Physiol. 2019;234:11960–11968. doi: 10.1002/jcp.27848. [DOI] [PubMed] [Google Scholar]
- 22.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Watafua M, Ejiofor JI, Musa A, Ahmad MH. Acacia sieberiana (Fabaceae) attenuates paracetamol and Bile Duct Ligation-Induced hepatotoxicity via modulation of biochemical and oxidative stress biomarkers. Front Pharmacol. 2022;13:959661. doi: 10.3389/fphar.2022.959661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed AMA, Rahman MA, Hossen MA, Reza ASMA, Islam MS, Rashid MM, Rafi MKJ, Siddiqui MTA, Al-Noman A, Uddin MN. Epiphytic Acampe ochracea orchid relieves paracetamol-induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomed Pharmacother. 2021;143:112215. doi: 10.1016/j.biopha.2021.112215. [DOI] [PubMed] [Google Scholar]
- 25.Korivi M, Hou CW, Huang CY, Lee SD, Hsu MF, Yu SH, Chen CY, Liu YY, Kuo CH. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid Based Complement Alternat Med. 2012;2012:932165. doi: 10.1155/2012/932165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CC, Lin TJ, Lu YF, Chen CC, Huang CY, Lin WT. Protective effects of L-arginine supplementation against exhaustive exercise-induced oxidative stress in young rat tissues. Chin J Physiol. 2009;52:306–315. doi: 10.4077/CJP.2009.AMH068. [DOI] [PubMed] [Google Scholar]
- 27.Cao L, Waldon D, Teffera Y, Roberts J, Wells M, Langley M, Zhao Z. Ratios of biliary glutathione disulfide (GSSG) to glutathione (GSH): a potential index to screen drug-induced hepatic oxidative stress in rats and mice. Anal Bioanal Chem. 2013;405:2635–2642. doi: 10.1007/s00216-012-6661-8. [DOI] [PubMed] [Google Scholar]
- 28.Bouhlali EDT, Derouich M, Hmidani A, Bourkhis B, Khouya T, Filali-Zegzouti Y, Alem C. Protective effect of Phoenix dactylifera L. seeds against paracetamol-induced hepatotoxicity in rats: a comparison with vitamin C. ScientificWorldJournal. 2021;2021:6618273. doi: 10.1155/2021/6618273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Z, Li Y, Tang Z, Song X, Jing F, Wu H, Lu B. Role of gambogenic acid in regulating PI3K/Akt/NF-kβ signaling pathways in rat model of acute hepatotoxicity. Biosci Biotechnol Biochem. 2021;85:520–527. doi: 10.1093/bbb/zbaa039. [DOI] [PubMed] [Google Scholar]
- 30.Steckling FM, Lima FD, Farinha JB, Rosa PC, Royes LFF, Cuevas MJ, Bresciani G, Soares FA, González-Gallego J, Barcelos RP. Diclofenac attenuates inflammation through TLR4 pathway and improves exercise performance after exhaustive swimming. Scand J Med Sci Sports. 2020;30:264–271. doi: 10.1111/sms.13579. [DOI] [PubMed] [Google Scholar]
- 31.Sinaga E, Fitrayadi A, Asrori A, Rahayu SE, Suprihatin S, Prasasty VD. Hepatoprotective effect of Pandanus odoratissimus seed extracts on paracetamol-induced rats. Pharm Biol. 2021;59:31–39. doi: 10.1080/13880209.2020.1865408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papastefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P. VEGF isoforms and receptors expression throughout acute acetaminophen-induced liver injury and regeneration. Arch Toxicol. 2007;81:729–741. doi: 10.1007/s00204-007-0201-x. [DOI] [PubMed] [Google Scholar]
- 33.Gokkaya EO, Yesilot S, Ozgocmen M, Aslankoc R, Aydin Acar C. Protective effects of resveratrol and avocado oil against paracetamol-induced hepatotoxicity in rats. Drug Chem Toxicol. 2022;45:2131–2139. doi: 10.1080/01480545.2021.1908716. [DOI] [PubMed] [Google Scholar]
- 34.Kuvandik G, Duru M, Nacar A, Yonden Z, Helvaci R, Koc A, Kozlu T, Kaya H, Sogüt S. Effects of erdosteine on acetaminophen-induced hepatotoxicity in rats. Toxicol Pathol. 2008;36:714–719. doi: 10.1177/0192623308320800. [DOI] [PubMed] [Google Scholar]
- 35.Vale AF, Ferreira HH, Benetti EJ, Rebelo ACS, Figueiredo ACR, Barbosa EC, Simões K. Antioxidant effect of the pequi oil (Caryocar brasiliense) on the hepatic tissue of rats trained by exhaustive swimming exercises. Braz J Biol. 2019;79:257–262. doi: 10.1590/1519-6984.180015. [DOI] [PubMed] [Google Scholar]
- 36.Sirotkin AV, Dekanová P, Harrath AH. FSH, oxytocin and IGF-I regulate the expression of sirtuin 1 in porcine ovarian granulosa cells. Physiol Res. 2020;69:461–466. doi: 10.33549/physiolres.934424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Gizawy HA, El-Haddad AE, Saadeldeen AM, Boshra SA. Tentatively identified (UPLC/T-TOF-MS/MS) compounds in the extract of Saussurea costus roots exhibit in vivo hepatoprotection via modulation of HNF-1α, Sirtuin-1, C/ebpα, miRNA-34a and miRNA-223. Molecules. 2022;27:2802. doi: 10.3390/molecules27092802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.BinMowyna MN, AlFaris NA. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm Biol. 2021;59:146–156. doi: 10.1080/13880209.2021.1877734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abd-Ella EM, El-Kott AF, El-Kenawy AE, Khalifa HS, Bin-Meferij MM, Alramlawy AM. Dehydroepiandrosterone protects against acetaminophen-induced liver damage in rats by upregulation of Bcl-2 and activation of sirt signalling. J Physiol Pharmacol. 2020:71. doi: 10.26402/jpp.2020.6.02. [DOI] [PubMed] [Google Scholar]
- 40.Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43:1589–1598. doi: 10.1007/s10753-020-01242-9. Erratum in: Inflammation 2021;44:2142. [DOI] [PubMed] [Google Scholar]
- 41.Bayod S, Del Valle J, Lalanza JF, Sanchez-Roige S, de Luxán-Delgado B, Coto-Montes A, Canudas AM, Camins A, Escorihuela RM, Pallàs M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol. 2012;47:925–935. doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Davari F, Alimanesh Z, Alimanesh Z, Salehi O, Hosseini SA. Effect of training and crocin supplementation on mitochondrial biogenesis and redox-sensitive transcription factors in liver tissue of type 2 diabetic rats. Arch Physiol Biochem. 2022;128:1215–1220. doi: 10.1080/13813455.2020.1762663. [DOI] [PubMed] [Google Scholar]
- 43.Morell CM, Fabris L, Strazzabosco M. Vascular biology of the biliary epithelium. J Gastroenterol Hepatol. 2013;28(Suppl 1 (01)):26–32. doi: 10.1111/jgh.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Küçükler S, Kandemir FM, Özdemir S, Çomaklı S, Caglayan C. Protective effects of rutin against deltamethrin-induced hepatotoxicity and nephrotoxicity in rats via regulation of oxidative stress, inflammation, and apoptosis. Environ Sci Pollut Res Int. 2021:62975–62990. doi: 10.1007/s11356-021-15190-w. [DOI] [PubMed] [Google Scholar]
- 45.Chen HJ, Liang TM, Lee IJ, Huang YT, Lin YL. Scutellariae radix suppresses LPS-induced liver endothelial cell activation and inhibits hepatic stellate cell migration. J Ethnopharmacol. 2013;150:835–842. doi: 10.1016/j.jep.2013.08.049. [DOI] [PubMed] [Google Scholar]