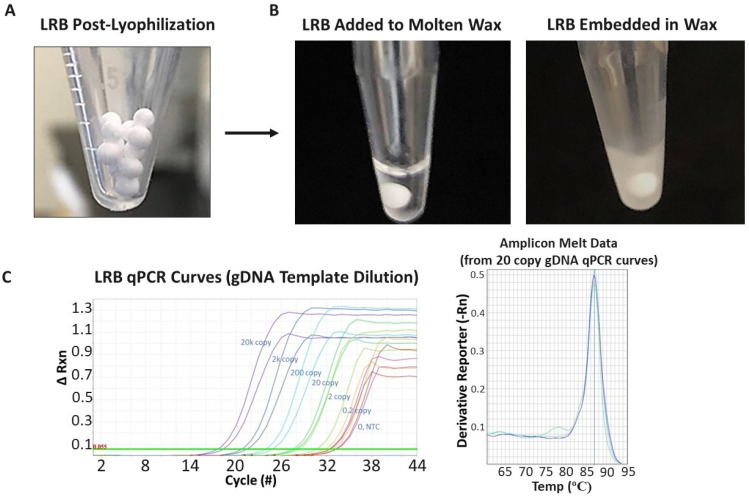
Figure 3.
(A) Image of typical LRB bead appearance after initial manufacturing stage. Successful bead manufacturing produces drug and/or PCR lyophilized reagent beads (LRBs) that are bright white and porous in appearance, yet are solid enough for further manipulation (i.e., moving to individual PCR vials and processing/covering in wax). (B) For the multiphase reaction chambers, the LRBs are added to a chamber containing molten wax (i.e., maintaining the wax-containing tube above the wax melting temperature; left). Once the LRB is added, the temperature is lowered, the wax rehardens, and the LRB is then embedded and protected within the wax phase. (C) (left) qPCR curves from initial runs in which a sample containing increasing concentrations of prepurified genomic E. coli DNA was added to the reaction chambers (over the wax); the wax was then melted; the LRB mixed with the added sample; and PCR performed. qPCR response was observed (i.e., decreased cycle number in which fluorescence increased over background; left) to the increased gDNA template concentrations (purple, 20,000 copies; black, 2000 copies; blue, 200 copies; green, 20 copies; light green, 2 copies; yellow, 0.2 copies; and red, 0 copies/no template control (NTC)). The melt curves (right) from the 20 gDNA copy qPCR curves show a clean/single product PCR result with a melting point (86.93 degrees Celsius) that matches the expectation for the E. coli primer set. Each colored line represents the melt curve from one PCR tube/reaction.