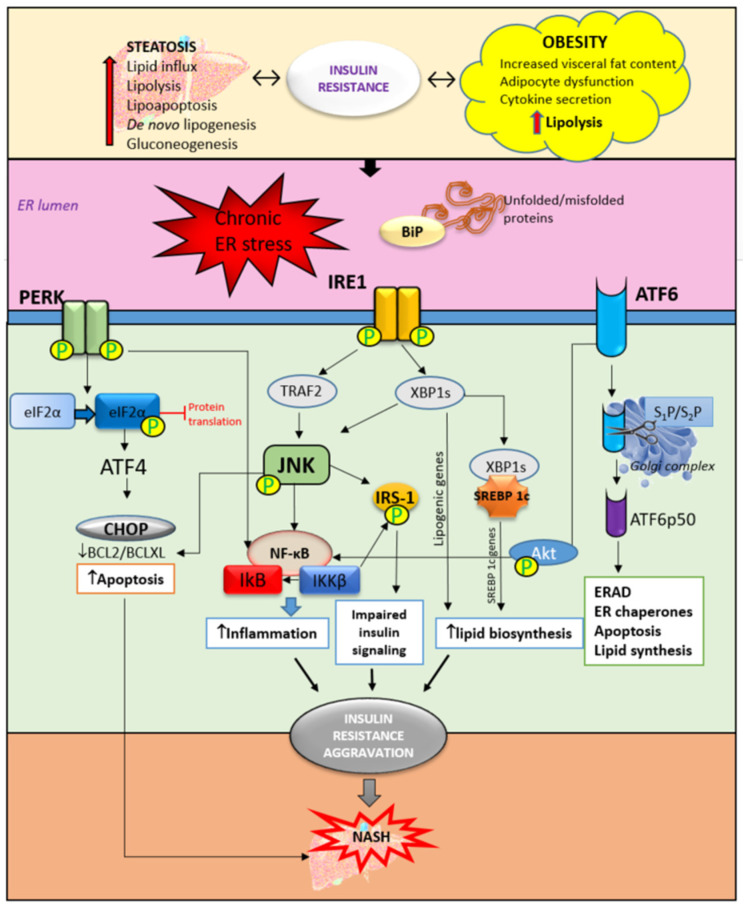
Figure 3.
The role of ER stress and UPR activation in insulin resistance and NASH development. Obesity and liver steatosis are bidirectionally connected with insulin resistance and are known to induce ER stress and consequently UPR activation. UPR is mediated through three pathways. The accumulation of unfolded and/or misfolded proteins trigger the activation of UPR. The activation and phosphorylation of IRE1, PERK, and ATF6 trigger an inflammatory response through JNK/NF-κB activation. Furthermore, impaired insulin signaling is mediated by the phosphorylation of IRS-1 mediated by JNK activation. In addition, ER stress triggers upregulation of the SREBP 1c receptor which contributes to lipid biosynthesis and accumulation. Accompanied by increased apoptosis of hepatocytes, these mechanisms are responsible, at least partly, for the insulin resistance aggravation contributing to NASH development. ER—endoplasmic reticulum, BiP—immunoglobulin heavy chain-binding protein, IRE1—inositol-requiring enzyme, PERK—PKR-like ER kinase, ATF6—activating transcription factor 6, eIF2α—eukaryotic initiation factor-2α, CHOP—C/EBP homologous protein, XBP1—XBP1, X-box binding protein 1, TRAF2—TNF receptor-associated factor 2, JNK—c-jun-N-terminal kinase, IRS1—insulin receptor substrate-1, ERAD—ER-associated degradation, SREBP—sterol regulatory element binding protein, IκB—inhibitor of nuclear factor kappa B, NF-κB—nuclear factor kappa B, and IKKβ—inhibitor of nuclear factor kappa-B kinase subunit beta.