Abstract
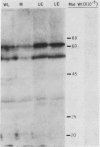
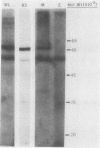
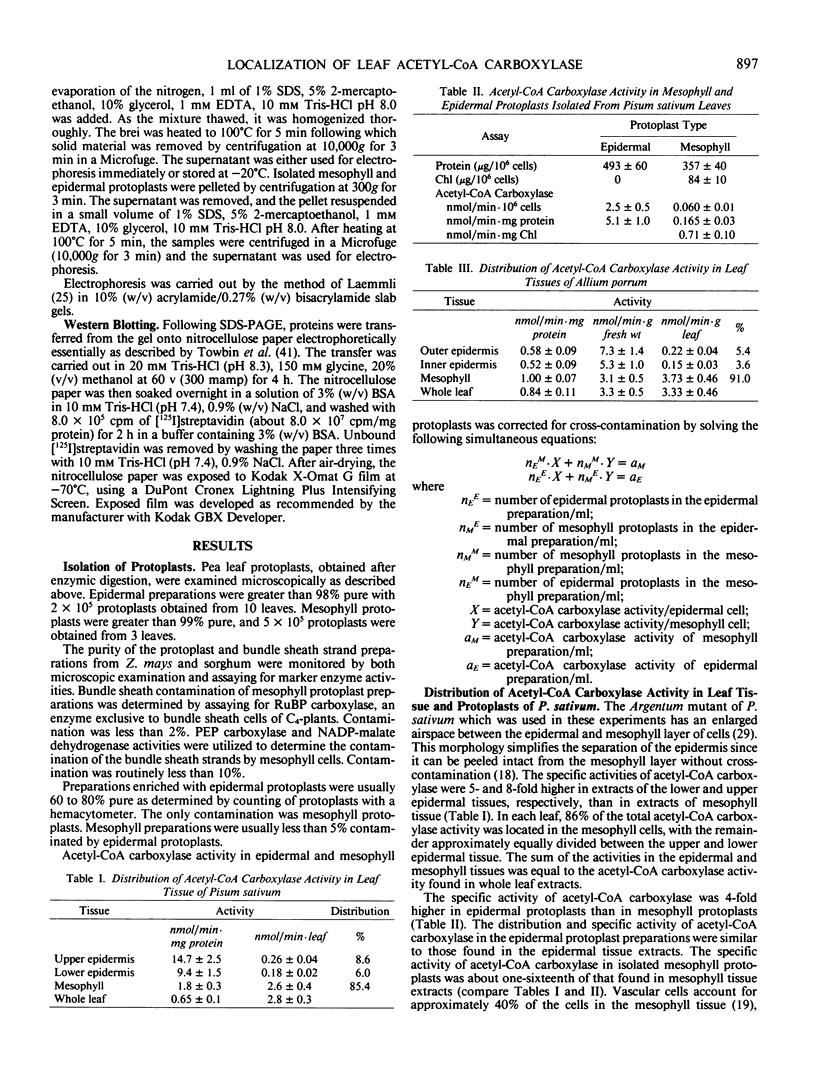
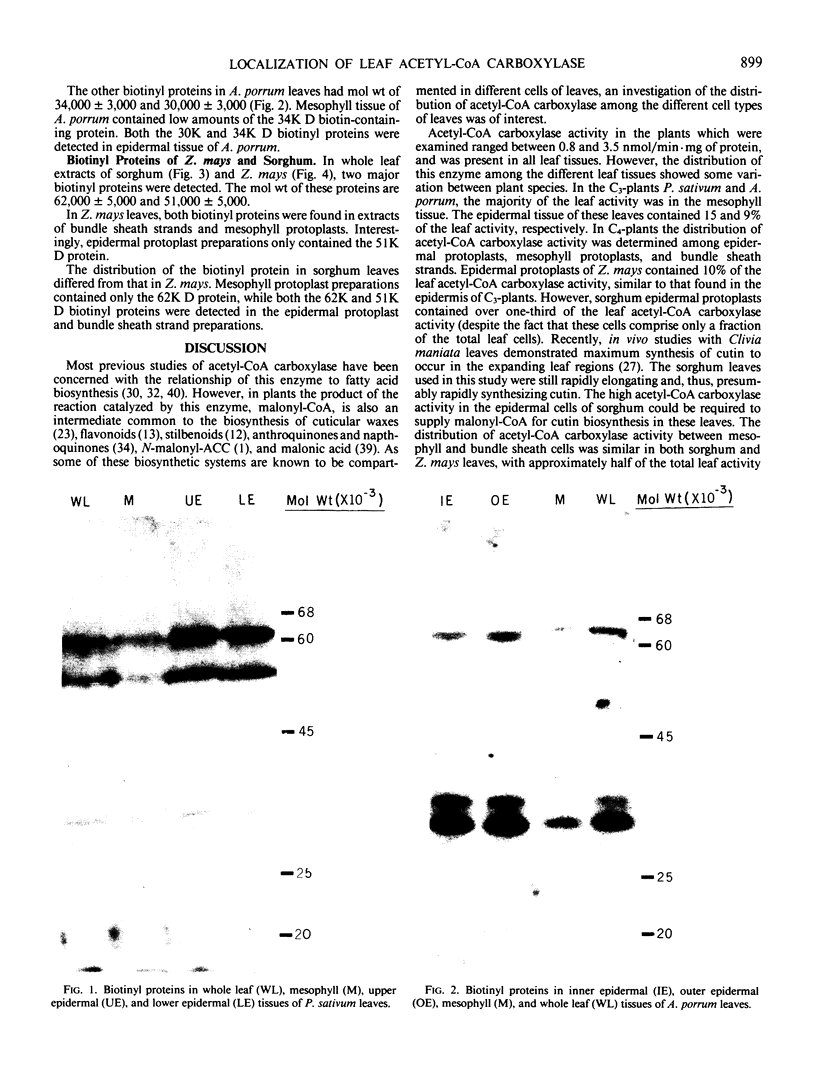
Acetyl-CoA carboxylase [acetyl-CoA—carbon dioxide ligase (ADP forming), EC 6.4.1.2] is a biotin-containing enzyme catalyzing the formation of malonyl-CoA. The tissue distribution of this enzyme was determined for leaves of C3- and C4-plants. The mesophyll tissues of the C3-plants Pisum sativum and Allium porrum contained 90% of the leaf acetyl-CoA carboxylase activity, with the epidermal tissues containing the remainder. Western blotting of proteins fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, using 125I-streptavidin as a probe, revealed biotinyl proteins of molecular weights 62,000, 51,000, and 32,000 in P. sativum and 62,000, 34,000, and 32,000 in A. porrum.
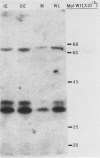
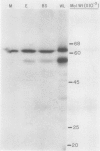
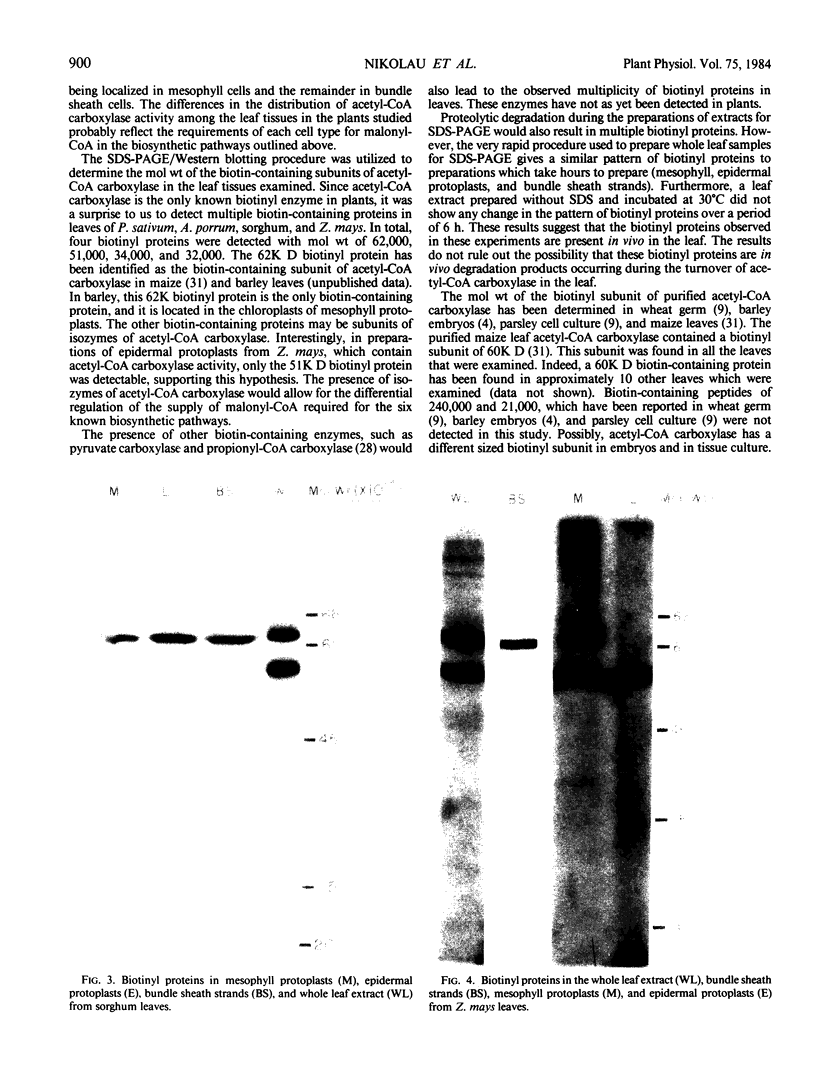
In the C4-plant sorghum, epidermal protoplasts, mesophyll protoplasts and strands of bundle sheath cells contained 35, 47, and 17%, respectively, of the total leaf acetyl-CoA carboxylase activity. In Zea mays leaves the respective figures were 10% for epidermal protoplasts, 56% for mesophyll protoplasts, and 32% for bundle sheath strands. Biotinyl proteins of molecular weights 62,000 and 51,000 were identified in leaves of sorghum and Z. mays.
The results are discussed with respect to each tissue's requirements for malonyl-CoA for various metabolic pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHAIET L., WOLF F. J. THE PROPERTIES OF STREPTAVIDIN, A BIOTIN-BINDING PROTEIN PRODUCED BY STREPTOMYCETES. Arch Biochem Biophys. 1964 Jul 20;106:1–5. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- Egin-Bühler B., Loyal R., Ebel J. Comparison of acetyl-CoA carboxylases from parsley cell cultures and wheat germ. Arch Biochem Biophys. 1980 Aug;203(1):90–100. doi: 10.1016/0003-9861(80)90156-3. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Heinstein P. F., Stumpf P. K. Fat metabolism in higher plants. 38. Properties of wheat germ acetyl coenzyme A carboxylase. J Biol Chem. 1969 Oct 10;244(19):5374–5381. [PubMed] [Google Scholar]
- Hoffman N. E., Fu J. R., Yang S. F. Identification and Metabolism of 1-(Malonylamino)cyclopropane-1-carboxylic Acid in Germinating Peanut Seeds. Plant Physiol. 1983 Jan;71(1):197–199. doi: 10.1104/pp.71.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrazdina G., Marx G. A., Hoch H. C. Distribution of Secondary Plant Metabolites and Their Biosynthetic Enzymes in Pea (Pisum sativum L.) Leaves : Anthocyanins and Flavonol Glycosides. Plant Physiol. 1982 Sep;70(3):745–748. doi: 10.1104/pp.70.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Henningsen K. W., Stumpf P. K., von Wettstein D. 6-Methylsalicylic acid synthesis by isolated barley chloroplasts. Eur J Biochem. 1971 Aug 16;21(3):334–338. doi: 10.1111/j.1432-1033.1971.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Knauf M., Stumpf P. K. Subcellular localization of acetyl-CoA synthetase in leaf protoplasts of Spinacia oleracea. Arch Biochem Biophys. 1981 Jul;209(2):441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynen F. New experiments of biotin enzymes. CRC Crit Rev Biochem. 1979 Dec;7(2):103–119. doi: 10.3109/10409237909105428. [DOI] [PubMed] [Google Scholar]
- Mohan S. B., Kekwick R. G. Acetyl-coenzyme A carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem J. 1980 Jun 1;187(3):667–676. doi: 10.1042/bj1870667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B. J., Hawke J. C. Purification and characterization of maize leaf acetyl-coenzyme A carboxylase. Arch Biochem Biophys. 1984 Jan;228(1):86–96. doi: 10.1016/0003-9861(84)90049-3. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Hawke J. C., Slack C. R. Acetyl-coenzyme A carboxylase in maize leaves. Arch Biochem Biophys. 1981 Oct 15;211(2):605–612. doi: 10.1016/0003-9861(81)90495-1. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprich N., Kindl H. Stilbene synthases and stilbenecarboxylate synthases, I Enzymatic synthesis of 3,5,4-trihydroxystilbene from p-coumaroyl coenzyme A and malonyl coenzyme A. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):165–172. [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Biosynthesis of malonate in roots of soybean seedlings. Plant Physiol. 1981 Nov;68(5):992–995. doi: 10.1104/pp.68.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Acetyl coenzyme a carboxylase activity in developing seedlings and chloroplasts of barley and its virescens mutant. Plant Physiol. 1981 Apr;67(4):655–661. doi: 10.1104/pp.67.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L., Piperno J. R., Walsh P. N. A rapid method for removal of [125I]iodide following iodination of protein solutions. Anal Biochem. 1980 Jul 15;106(1):118–122. doi: 10.1016/0003-2697(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Wurtele E. S., Thayer S. S., Conn E. E. Subcellular Localization of a UDP-Glucose:Aldehyde Cyanohydrin beta-Glucosyl Transferase in Epidermal Plastids of Sorghum Leaf Blades. Plant Physiol. 1982 Dec;70(6):1732–1737. doi: 10.1104/pp.70.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]