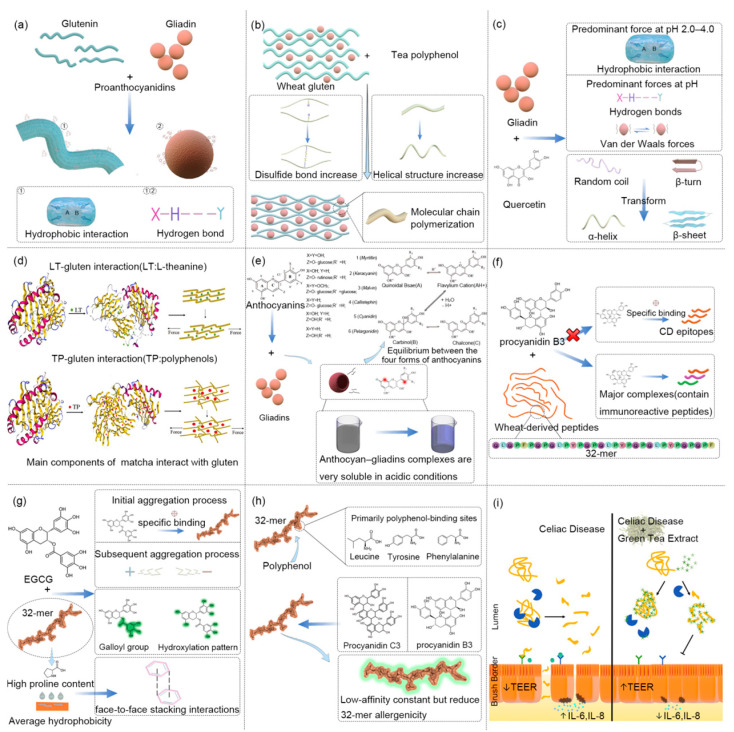
Figure 5.
Interaction of gluten and dietary polyphenols. (a) Non-covalent linkage of proanthocyanidins to wheat proteins [115]. (b) Tea polyphenols promote structural changes and polymerization of wheat gluten [117]. (c) Different non-donor linkages between quercetin and gliadins at different pH and the structure of gliadin changes [118]. (d) Tea polyphenols form ternary hydrogen bonds with wheat protein to stabilize its network structure [116] (reproduced with permission from Ref. [116]. Copyright publisher Elsevier). (e) Anthocyans change the secondary structure of gliadin [119] (reproduced with permission from Ref. [119]. Copyright publisher Elsevier). (f–h) Interaction mechanism of proanthocyanidin B3, EGCG, green tea polyphenols, proanthocyanidin monomers, polymers, and wheat protein peptides [121,122] (reproduced with permission from Refs. [121,122]. Copyright publisher Elsevier). (i) The complexes of green tea polyphenols and gliadins regulate inflammation [123] (reproduced with permission from Ref. [123]. Copyright publisher Wiley Company).