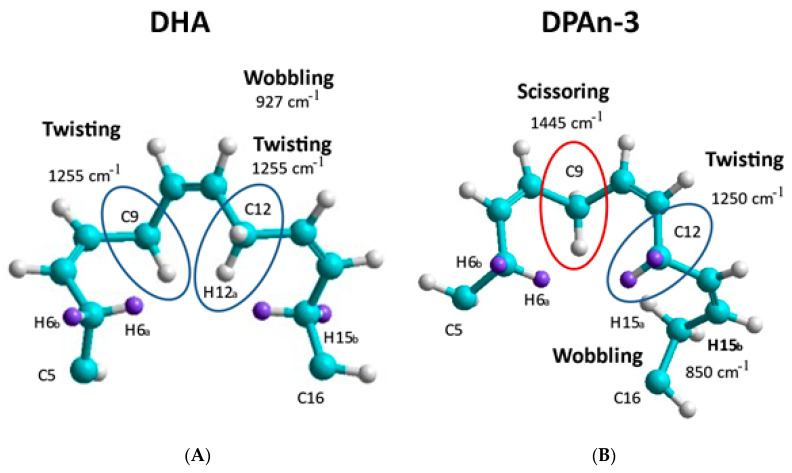
Figure 3.
Vibrational modes generated by the computation chemistry of DHA and DPAn-3 are compared. (A) DHA, sites C5-C16, and (B) DPAn-3, sites C5-C16. Spontaneous shape-change behaviors are illustrated by comparing sections of the two molecules. Additionally, with DHA, three double bonds can be planar, whereas with DPAn-3, there are only two. The greater planarity is also consistent with the potential for the absorption of vibrational energy. Light blue atoms are carbon, white atoms are hydrogen. The purple atoms are also hydrogen highlighted to show in DHA twist at C5 is very different from C5 in DPAn-3. Adapted from [39].