Figure 4.
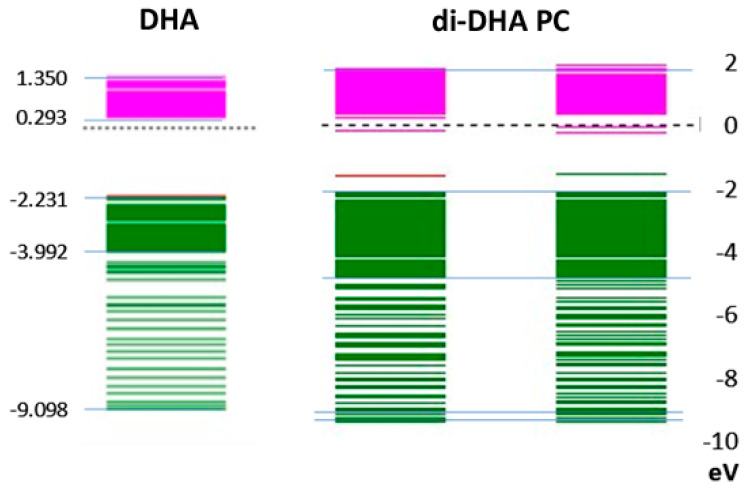
DHA and di-DHA PC spin orbitals are compared. There is a clear distinction between the molecular properties of DHA and di-DHA PC. The broadening from −2 to −4 to −5 ev and at −9 to −10 ev in the di-DHA is particularly noticeable, so much so that it raises the question as to whether it is specifically engaged in the energy transductions as it will likely be more sensitive than the single DHA. Moreover, the left-hand arm has more broadening from −6 to −9 ev, which could be significant. The green color is for spin orbital below the molecular plane. The electrons on protons below the plane are forced closer together due to the repeating cis-bonds. The redundancy in electron spin orbital methylene site to methylene site are similar but not identical. The pink color corresponds to electron spins at sites above the molecular plane.