Abstract
Soybean (Glycine max [L.] Merr.) germplasm, isogenic except for loci controlling male-sterility (ms1) and nodulation (rj1) was utilized to investigate the effects of reproductive tissue development and nitrogen source on the initiation of monocarpic senescence. The experimental genotypes (Ms1Rj1, Ms1rj1, ms1Rj1, and ms1rj1, were selected from a cross between N69-2774 and N59-5259, and were inbred to the F5 generation. Green-house-grown plants were collected during the period of flowering (77 days after transplanting) until maturity (147 days after transplanting). Leaf tissues from the respective genotypes were analyzed at the various harvest dates for RNA, phenolic, and chlorophyll concentrations; acid protease activity; polypeptide banding patterns of chloroplast thylakoids; and chloroplastic ultrastructure.
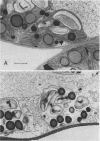
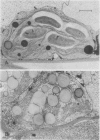
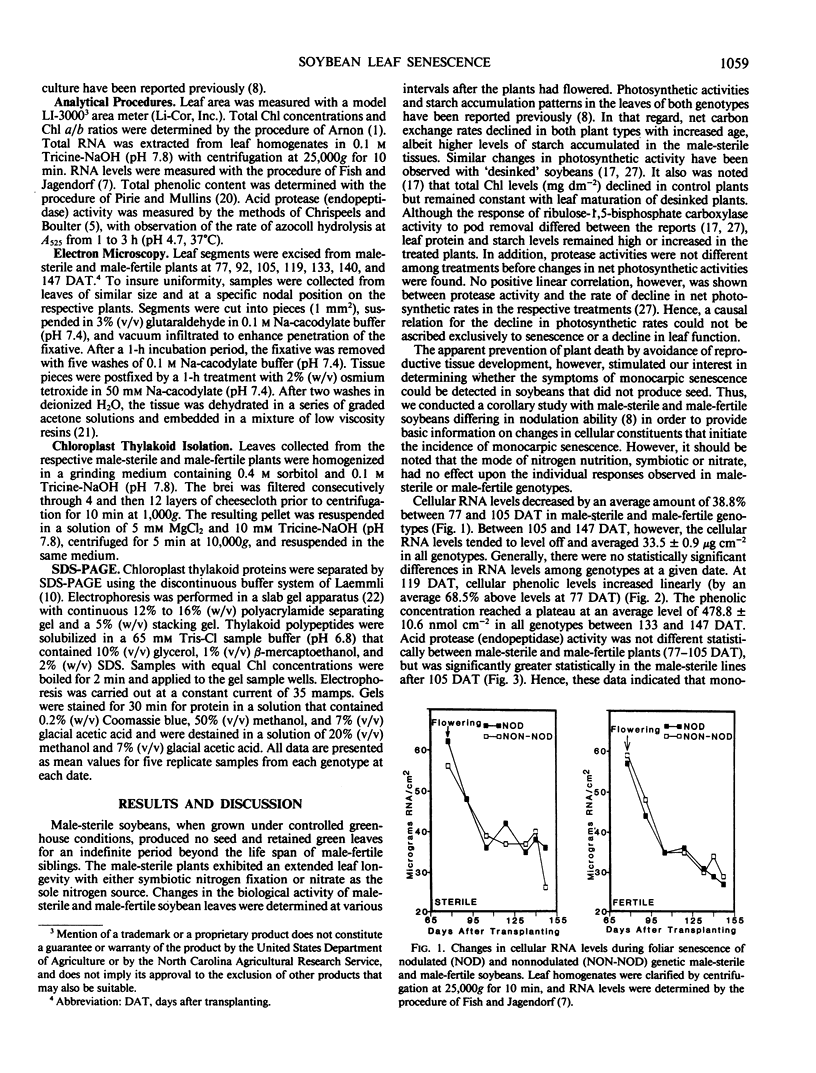
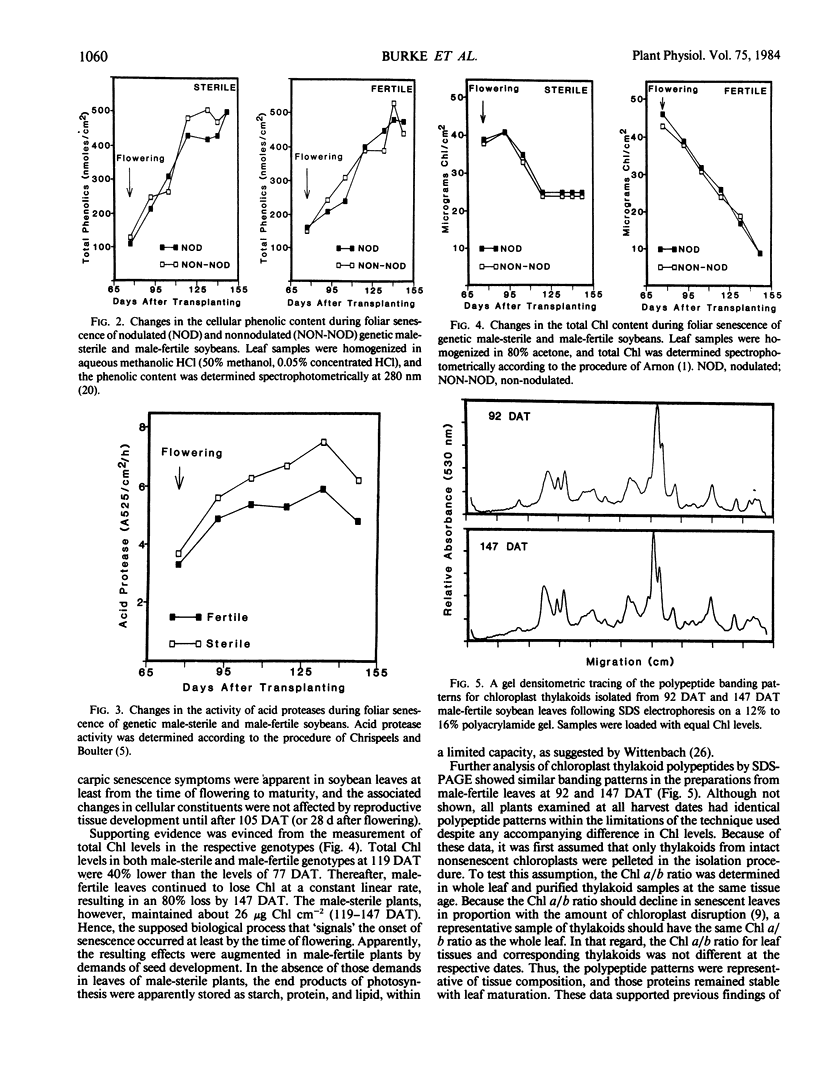
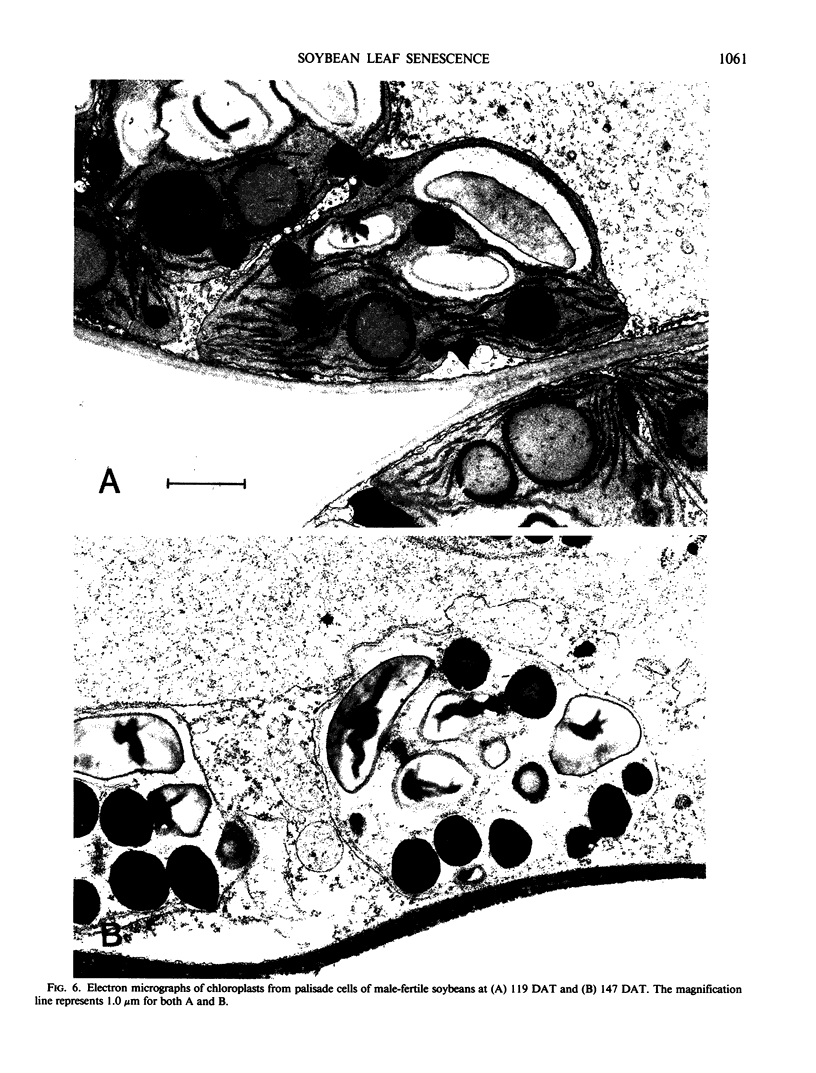
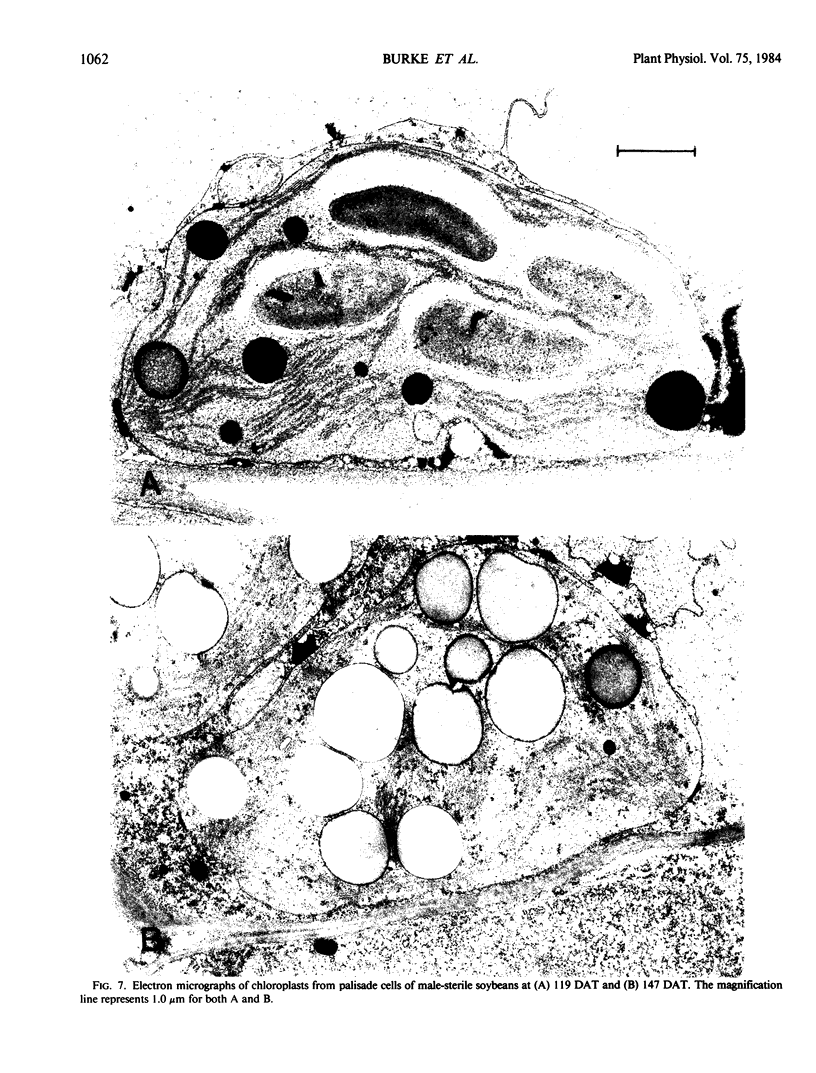
Regardless of nitrogen source, total chlorophyll concentrations declined between 77 and 119 days after transplanting, resulting in a 40% loss of chlorophyll per square centimeter in all genotypes. Leaf chlorophyll levels continued to decline at a constant rate in male-fertile genotypes, but remained at a constant level (26 micrograms chlorophyll per square centimeter) in male-sterile genotypes, for the remainder of the study. With increased leaf age, a gradual disruption of thylakoid structures was observed, particularly in chloroplasts from the male-fertile genotypes. Chloroplasts from the male-sterile genotypes appeared to lose starch grains but increased their number of chloroplastic lipid bodies with leaf aging. These data suggest that monocarpic senescence in soybeans was initiated at or before flowering. Although reproductive tissue development probably augmented the process, the response attributed to seed formation was not apparent until the mid-pod fill stage (119 days after transplanting). All genotypes had similar changes in other cellular components that are recognized as indicators of plant senescence regardless of whether the plants produced seed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Arntzen C. J. The Occurence of delta-Tocopherylquinone in Higher Plants and Its Relation to Senescence. Plant Physiol. 1969 Apr;44(4):591–598. doi: 10.1104/pp.44.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp P. J., Huber S. C., Burke J. J., Moreland D. E. Biochemical Changes that Occur during Senescence of Wheat Leaves : I. Basis for the Reduction of Photosynthesis. Plant Physiol. 1982 Dec;70(6):1641–1646. doi: 10.1104/pp.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L., Jagendorf A. T. A method for enzymic extraction and the measurement of chloroplast RNA. Plant Physiol. 1980 Apr;65(4):746–750. doi: 10.1104/pp.65.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Wilson R. F., Burton J. W. Studies on Genetic Male-Sterile Soybeans : II. Effect of Nodulation on Photosynthesis and Carbon Partitioning in Leaves. Plant Physiol. 1983 Nov;73(3):713–717. doi: 10.1104/pp.73.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leopold A. C., Niedergang-Kamien E., Janick J. Experimental Modification of Plant Senescence. Plant Physiol. 1959 Sep;34(5):570–573. doi: 10.1104/pp.34.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindoo S. J., Noodén L. D. Studies on the behavior of the senescence signal in anoka soybeans. Plant Physiol. 1977 Jun;59(6):1136–1140. doi: 10.1104/pp.59.6.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M. H., Brun W. A., Brenner M. L. Effects of Sink Removal on Photosynthesis and Senescence in Leaves of Soybean (Glycine max L.) Plants. Plant Physiol. 1978 Mar;61(3):394–397. doi: 10.1104/pp.61.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A., Mullins M. G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic Acid. Plant Physiol. 1976 Oct;58(4):468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Thomas H., Stoddart J. L. Separation of Chlorophyll Degradation from Other Senescence Processes in Leaves of a Mutant Genotype of Meadow Fescue (Festuca pratensis L.). Plant Physiol. 1975 Sep;56(3):438–441. doi: 10.1104/pp.56.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Burton J. W., Buck J. A., Brim C. A. Studies on Genetic Male-Sterile Soybeans: I. Distribution of Plant Carbohydrate and Nitrogen during Development. Plant Physiol. 1978 May;61(5):838–841. doi: 10.1104/pp.61.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 1982 Nov;70(5):1544–1548. doi: 10.1104/pp.70.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]