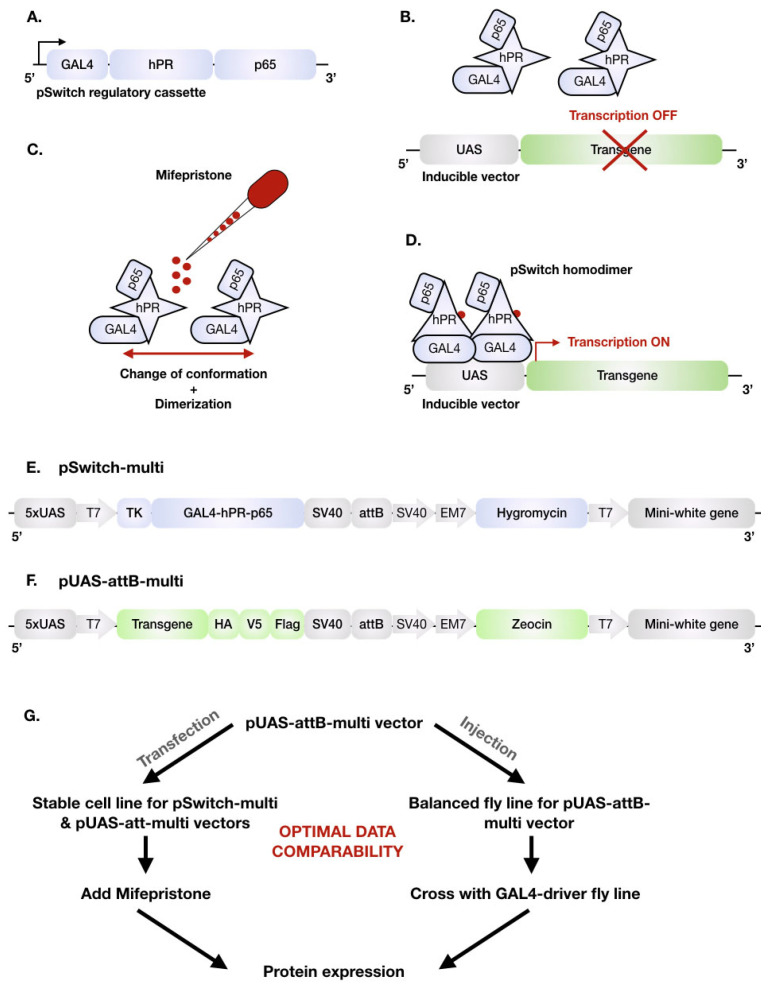
Figure 1.
Schematic illustration of the adapted pSwitch system. (A) Simplified scheme of the pSwitch regulatory cassette containing the yeast specific GAL4 DNA binding domain, the truncated human progesterone receptor ligand binding domain (hPR), and an activation domain from the human NF-KB p65 protein (p65). This cassette encodes for the (B) pSwitch regulatory fusion protein in a monomeric inactive state. (C) By adding Mifepristone (red), a human progesterone receptor antagonist, the pSwitch fusion protein undergoes a conformational change leading to homodimerization. (D) The homodimer binds to the yeast UAS sequence to activate transgene expression. We adapted the GeneSwitch™ system by generating (E) the inducible pSwitch-multi vector containing five UAS (5 × UAS), a T7 promoter, a TK minimal promoter, the pSwitch regulatory cassette (GAL4-hPR-p65), a simian virus 40 polyadenylation site (SV40), an attB sequence for site-directed insertion, an SV40 promoter, an EM7 promoter, an Hygromycin resistance gene, and the mini-white gene. (F) The inducible pUAS-attB-multi plasmid contains the same grey features as the pSwitch regulatory vector, the cDNA sequence of the transgene flanked by three tags (HA, V5 and Flag) and a Zeocin resistance gene. (G) Schematic representation of the experimental layout for which expression of the transgene in the pUAS-attB-multi vector can now be controlled in cells and flies using Mifepristone or GAL4 activation, respectively, to ensure optimal data comparability without the need for subcloning.