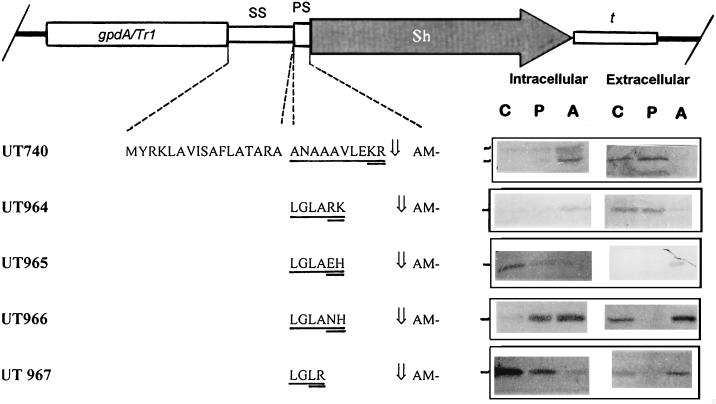
FIG. 4.
Effect of pAPMSF on formation and secretion by T. reesei of the phleomycin-binding protein fused to different proprotein sequences. The N-terminal amino acid of the secreted protein was determined by Edman degradation and is marked by an arrow. The UT numbers of the respective recombinant strains are given in the left margin. The construct is shown on top (gpdA/Tr1, promoter; SS, signal sequence; PS, prosequence; Sh, the phleomycin-binding protein-encoding sequence; t, TrpC terminator). Sequences differing between the different constructs are underlined, and the putative cleavage targets are double underlined. Detection of intracellular and extracellular levels of ShBLE (13.7 kDa) by SDS-PAGE and immunostaining with a polyclonal antibody are shown to the right of the amino acid sequences. Columns: C, control (no inhibitor added); P, PMSF; A, pAPMSF. The markers on the left of the blots indicate the positions of the mature proteins. For intracellular samples, equal amounts (40 μg) of protein were loaded onto the gels; equal volumes (20 μl) of the culture broth were loaded for extracellular samples. The blots shown are typical for the results obtained in three independent experiments.